
Diclofenac, sold under the brand name Voltaren among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammatory diseases such as gout. It can be taken orally, inserted rectally as a suppository, injected intramuscularly, injected intravenously, applied to the skin topically, or through eye drops. Improvements in pain last up to eight hours. It is also available as the fixed-dose combination diclofenac/misoprostol (Arthrotec) to help protect the stomach.

Prednisolone is a corticosteroid, a steroid hormone used to treat certain types of allergies, inflammatory conditions, autoimmune disorders, and cancers, electrolyte imbalances and skin conditions. Some of these conditions include adrenocortical insufficiency, high blood calcium, rheumatoid arthritis, dermatitis, eye inflammation, asthma, multiple sclerosis, and phimosis. It can be taken by mouth, injected into a vein, used topically as a skin cream, or as eye drops. It differs from the similarly named prednisone in having a hydroxyl at the 11th carbon instead of a ketone.

Dry eye syndrome, also known as keratoconjunctivitis sicca, is the condition of having dry eyes. Symptoms include dryness in the eye, irritation, redness, discharge, blurred vision, and easily fatigued eyes. Symptoms range from mild and occasional to severe and continuous. Dry eye syndrome can lead to blurred vision, instability of the tear film, increased risk of damage to the ocular surface such as scarring of the cornea, and changes in the eye including the neurosensory system.

Ketorolac, sold under the brand name Toradol among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain. Specifically it is recommended for moderate to severe pain. Recommended duration of treatment is less than six days, and in Switzerland not more than seven days. It is used by mouth, by nose, by injection into a vein or muscle, and as eye drops. Effects begin within an hour and last for up to eight hours. Ketorolac also has antipyretic (fever-reducing) properties.
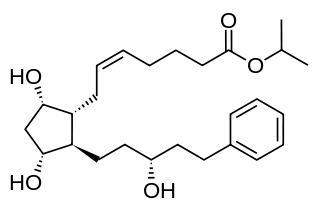
Latanoprost, sold under the brand name Xalatan among others, is a medication used to treat increased pressure inside the eye. This includes ocular hypertension and open-angle glaucoma. Latanaprost is applied as eye drops to the eyes. Onset of effects is usually within four hours, and they last for up to a day.

Artificial tears are lubricating eye drops used to relieve dryness and irritation of the ocular surface. Dry eye syndrome is a common ocular surface disorder and is characterized by disruption of the tear film and increased inflammation.

Corneal cross-linking (CXL) with riboflavin (vitamin B2) and UV-A light is a surgical treatment for corneal ectasia such as keratoconus, PMD, and post-LASIK ectasia.
Dorzolamide/timolol, sold under the brand name Cosopt among others, is a medication used to treat high pressure inside the eye including glaucoma. It is a combination of dorzolamide hydrochloride and timolol maleate. It may be used when a beta blocker, like timolol, is not sufficient alone. It is used as an eye drop.

Loteprednol is a topical corticosteroid used to treat inflammations of the eye. It is marketed by Bausch and Lomb as Lotemax and Loterex.

Difluprednate, sold under the brand name Durezol, is a corticosteroid used for the treatment of post-operative ocular inflammation and pain.

Endophthalmitis, or endophthalmia, is inflammation of the interior cavity of the eye, usually caused by an infection. It is a possible complication of all intraocular surgeries, particularly cataract surgery, and can result in loss of vision or loss of the eye itself. Infection can be caused by bacteria or fungi, and is classified as exogenous, or endogenous. Other non-infectious causes include toxins, allergic reactions, and retained intraocular foreign bodies. Intravitreal injections are a rare cause, with an incidence rate usually less than 0.05%.

Nepafenac, sold under the brand name Nevanac among others, is a nonsteroidal anti-inflammatory drug (NSAID), usually sold as a prescription eye drop 0.1% solution (Nevanac) or 0.3% solution (Ilevro). It is used to treat pain and inflammation associated with cataract surgery. Nepafenac is a prodrug of amfenac, an inhibitor of COX-1 and COX-2 activity.

N-Acetylcarnosine (NAC) is a naturally occurring compound chemically related to the dipeptide carnosine. The NAC molecular structure is identical to carnosine with the exception that it carries an additional acetyl group. The acetylation makes NAC more resistant to degradation by carnosinase, an enzyme that breaks down carnosine to its constituent amino acids, beta-alanine and histidine.

Besifloxacin (INN/USAN) is a fourth-generation fluoroquinolone antibiotic. The marketed compound is besifloxacin hydrochloride. It was developed by SSP Co. Ltd., Japan, and designated SS734. SSP licensed U.S. and European rights to SS734 for ophthalmic use to InSite Vision Incorporated in 2000. InSite Vision developed an eye drop formulation (ISV-403) and conducted preliminary clinical trials before selling the product and all rights to Bausch & Lomb in 2003.

ISTA Pharmaceuticals, Inc., was a US-based pharmaceutical company that specialized in ophthalmic pharmaceutical products and discovers, develops, and markets therapies for inflammation, ocular pain, glaucoma, allergy, and dry eye. ISTA was acquired by Bausch & Lomb, an eye care company, on March 26, 2012. Under the deal, Bausch & Lomb have agreed to pay $9.10 per share for ISTA, bringing the total value of the acquisition to $500 million. In 2012, Valeant Pharmaceuticals withdrew its $360 million offer.

Alcaftadine, sold under the brand name Lastacaft, is an antihistamine used to help prevent itching of the eyes. It is an H1 histamine receptor antagonist. It is given as an drops in the eye.
Micro-invasive glaucoma surgery (MIGS) is the latest advance in surgical treatment for glaucoma, which aims to reduce intraocular pressure by either increasing outflow of aqueous humor or reducing its production. MIGS comprises a group of surgical procedures which share common features. MIGS procedures involve a minimally invasive approach, often with small cuts or micro-incisions through the cornea that causes the least amount of trauma to surrounding scleral and conjunctival tissues. The techniques minimize tissue scarring, allowing for the possibility of traditional glaucoma procedures such as trabeculectomy or glaucoma valve implantation to be performed in the future if needed.
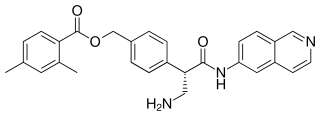
Netarsudil, sold under the brand name Rhopressa among others, is a medication for the treatment of glaucoma. In the United States, in December 2017, the Food and Drug Administration (FDA) approved a 0.02% ophthalmic solution for the lowering of elevated intraocular pressure in people with open-angle glaucoma or ocular hypertension. The European Medicines Agency approved it in 2019 for the same uses under the brand name Rhokiinsa.

Phacolytic glaucoma (PG) is a form of glaucoma which is caused due to a leaking mature or immature cataract. Inflammatory glaucoma which occurs in phacolysis is a condition which is a result of the leakage of protein within the lens into the capsule of a mature or hyper mature cataract and involves a simple procedure to be cured that is referred to as cataract extraction.
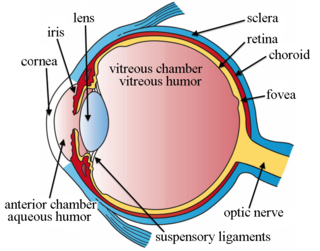
Intravitreal injection is the method of administration of drugs into the eye by injection with a fine needle. The medication will be directly applied into the vitreous humor. It is used to treat various eye diseases, such as age-related macular degeneration (AMD), diabetic retinopathy, and infections inside the eye such as endophthalmitis. As compared to topical administration, this method is beneficial for a more localized delivery of medications to the targeted site, as the needle can directly pass through the anatomical eye barrier and dynamic barrier. It could also minimize adverse drug effects on other body tissues via the systemic circulation, which could be a possible risk for intravenous injection of medications. Although there are risks of infections or other complications, with suitable precautions throughout the injection process, chances for these complications could be lowered.




















