
A lactam is a cyclic amide, formally derived from an amino carboxylic acid through cyclization reactions. [1] The term is a portmanteau of the words lactone + amide .

A lactam is a cyclic amide, formally derived from an amino carboxylic acid through cyclization reactions. [1] The term is a portmanteau of the words lactone + amide .
Greek prefixes in alphabetical order indicate ring size.
| Ring size (number of atoms in the ring) | Systematic name | IUPAC name | Common name (s) | Structure |
|---|---|---|---|---|
| 3 | α-Lactam | Aziridin-2-one | α-Acetolactam |  |
| 4 | β-Lactam | Azetidin-2-one | β-Propiolactam | 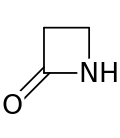 |
| 5 | γ-Lactam | Pyrrolidin-2-one |
|  |
| 6 | δ-Lactam | Piperidin-2-one |
|  |
| 7 | ε-Lactam | Azepan-2-one |
|  |
This ring-size nomenclature stems from the fact that hydrolysis of an α-lactam gives an α-amino acid and that of a β-Lactam gives a β-amino acid, and so on.
General synthetic methods are used for the organic synthesis of lactams.
Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement.
Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclohexanone with hydrazoic acid, forms ε - Caprolactum, which upon treatment with excess acid forms Cardiazole, a heart stimulant.
Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the same molecule. Lactamization is most efficient in this way if the product is a γ-lactam. For example, Fmoc-Dab(Mtt)-OH, although its side-chain amine is sterically protected by extremely bulky 4-Methyltrityl (Mtt) group, the amine can still intramolecularly couple with the carboxylic acid to form a γ-lactam. This reaction almost finished within 5 minutes with many coupling reagents (e.g. HATU and PyAOP). [2]
Lactams form from intramolecular attack of linear acyl derivatives from the nucleophilic abstraction reaction.
An iminium ion reacts with a halonium ion formed in situ by reaction of an alkene with iodine. [3]

Lactams form by copper-catalyzed 1,3-dipolar cycloaddition of alkynes and nitrones in the Kinugasa reaction
Diels-Alder reaction between cyclopentadiene and chlorosulfonyl isocyanate (CSI) can be utilized to obtain both β- as well as γ-lactam. At lower temp (−78 °C), β-lactam is the preferred product. At optimum temperatures, a highly useful γ-lactam known as Vince Lactam [4] is obtained. [5]

A lactim is a cyclic imidic acid compound characterized by an endocyclic carbon-nitrogen double bond. They are formed when lactams undergo tautomerization.
