 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xalatan, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697003 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical eye drop |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Activation by ester hydrolysis, deactivation by beta oxidation |
| Onset of action | 3–4 hours |
| Elimination half-life | 17 minutes (plasma) |
| Duration of action | ≥ 24 hours |
| Excretion | Mainly via kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.162.178 |
| Chemical and physical data | |
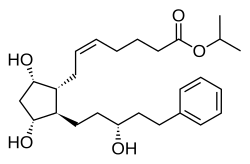
| Formula | C26H40O5 |
| Molar mass | 432.601 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Latanoprost, sold under the brand name Xalatan among others, is a medication used to treat increased pressure inside the eye (intraocular pressure). [5] This includes ocular hypertension and open-angle glaucoma. [5] Latanaprost is applied as eye drops to the eyes. [5] Onset of effects is usually within four hours, and they last for up to a day. [5]
Contents
- Medical uses
- Open-angle glaucoma
- Closed-angle glaucoma
- Adverse effects
- Pregnancy
- Interactions
- Pharmacology
- Mechanism of action
- Pharmacokinetics
- Chemistry
- Stability
- Society and culture
- Legal status
- Brand names
- Cosmetic use
- See also
- References
Common side effects include blurry vision, redness of the eye, itchiness, and darkening of the iris. [5] Latanoprost is in the prostaglandin analogue family of medications. [5] It works by increasing the outflow of aqueous fluid from the eyes through the uveoscleral tract. [6]
Latanoprost was approved for medical use in the United States and the European Union in 1996. [5] [3] It is on the World Health Organization's List of Essential Medicines. [7] Latanoprost is available as a generic medication. [8] In 2023, it was the 67th most commonly prescribed medication in the United States, with more than 9 million prescriptions. [9] [10] It is available as a fixed-dose combination with netarsudil as netarsudil/latanoprost and with timolol as latanoprost/timolol.


