
An analgesic or painkiller is any member of the group of drugs used to achieve analgesia, relief from pain.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are members of a drug class that reduces pain, decreases fever, prevents blood clots, and in higher doses, decreases inflammation. Side effects depend on the specific drug but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease.

Naproxen, sold under the brand name Aleve among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain, menstrual cramps, inflammatory diseases such as rheumatoid arthritis, and fever. It is taken orally. It is available in immediate and delayed release formulations. Onset of effects is within an hour and last for up to twelve hours.

Orphenadrine is an anticholinergic drug of the ethanolamine antihistamine class; it is closely related to diphenhydramine. It is used to treat muscle pain and to help with motor control in Parkinson's disease, but has largely been superseded by newer drugs. This substance is considered a dirty drug due to its multiple mechanism of action in different pathways. It was discovered and developed in the 1940s.

Antimigraine drugs are medications intended to reduce the effects or intensity of migraine headache. They include drugs for the treatment of acute migraine symptoms as well as drugs for the prevention of migraine attacks.

Tenoxicam, sold under the brand name Mobiflex among others, is a nonsteroidal anti-inflammatory drug (NSAID). It is used to relieve inflammation, swelling, stiffness, and pain associated with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, tendinitis, bursitis, and periarthritis of the shoulders or hips.
Naproxcinod (nitronaproxen) is a nonsteroidal anti-inflammatory drug (NSAID) developed by the French pharmaceutical company NicOx. It is a derivative of naproxen with a nitroxybutyl ester to allow it to also act as a nitric oxide (NO) donor. This second mechanism of action makes naproxcinod the first in a new class of drugs, the cyclooxygenase inhibiting nitric oxide donators (CINODs), that are hoped to produce similar analgesic efficacy to traditional NSAIDs, but with less gastrointestinal and cardiovascular side effects.
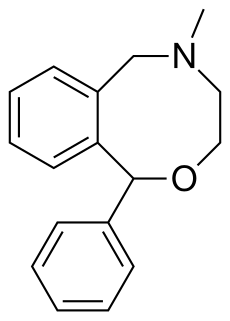
Nefopam, sold under the brand name Acupan among others, is a centrally acting, non-opioid painkilling medication, that is primarily used to treat moderate to severe pain.
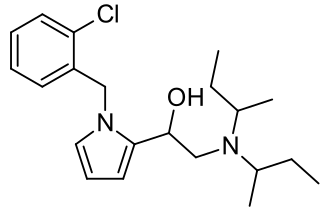
Viminol is an opioid analgesic developed by a team at the drug company Zambon in the 1960s. Viminol is based on the α-pyrryl-2-aminoethanol structure, unlike any other class of opioids.
An equianalgesic chart is a conversion chart that lists equivalent doses of analgesics. Equianalgesic charts are used for calculation of an equivalent dose between different analgesics. Tables of this general type are also available for NSAIDs, benzodiazepines, depressants, stimulants, anticholinergics and others as well.

Eltoprazine (DU-28,853) is a drug of the phenylpiperazine class which is a serenic or antiaggressive agent. It acts as an agonist at the 5-HT1A and 5-HT1B receptors and as an antagonist at the 5-HT2C receptor. It is closely related to fluprazine and batoprazine, which are similarly acting drugs.
An enantiopure drug is a pharmaceutical that is available in one specific enantiomeric form. Most biological molecules are present in only one of many chiral forms, so different enantiomers of a chiral drug molecule bind differently to target receptors. One enantiomer of a drug may have a desired beneficial effect while the other may cause serious and undesired side effects, or sometimes even beneficial but entirely different effects. Advances in industrial chemical processes have made it economical for pharmaceutical manufacturers to take drugs that were originally marketed as a racemic mixture and market the individual enantiomers, either by specifically manufacturing the desired enantiomer or by resolving a racemic mixture. On a case-by-case basis, the U.S. Food and Drug Administration (FDA) has allowed single enantiomers of certain drugs to be marketed under a different name than the racemic mixture. Also case-by-case, the United States Patent Office has granted patents for single enantiomers of certain drugs. The regulatory review for marketing approval and for patenting is independent, and differs country by country.

BDPC (systematic name 4-(4-bromophenyl)-4-(dimethylamino)-1-(2-phenylethyl)cyclohexanol; also known as bromadol) is a potent narcotic analgesic with a distinctive arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. Initial studies estimated that it was around 10,000 times the strength of morphine in animal models. However, later studies using more modern techniques assigned a value of 504 times the potency of morphine for the more active trans-isomer. This drug was first seized along with three kilograms of acetylfentanyl in an April 25, 2013 police action in Montreal, Canada, and has reportedly continued to be available on the designer drug black market internationally. Analogues where the para-bromine is replaced by chlorine or CH3 retain similar activity, as does the meta-hydroxy derivative.

1-Phenylpiperazine is a simple chemical compound featuring a phenyl group bound to a piperazine ring. The suffix ‘-piprazole’ is sometimes used in the names of drugs to indicate they belong to this class.

Elopiprazole is an antipsychotic drug of the phenylpiperazine class which was never marketed.

TROX-1 is a drug which acts as a potent blocker of the Cav2 type calcium channels. It was developed as a potential analgesic after the discovery that the selective Cav2.2 blocker ziconotide is an active analgesic with similar efficacy to strong opioid drugs. Unlike ziconotide, TROX-1 is not so selective, and also blocks the Cav2.1 and Cav2.3 calcium channel subtypes, but it has the great advantage of being orally active, whereas ziconotide must be administered intrathecally, by injection into the spinal fluid. In animal studies of TROX-1, analgesic effects were observed with similar efficacy to NSAIDs such as naproxen or diclofenac, and anti-allodynia effects equivalent to pregabalin or duloxetine.

MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl)piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer. It has been used as a lead compound from which a large family of potent opioid drugs have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes. Fluorinated derivates of MT-45 are potent mu opioid receptor agonists and one of its main metabolites also activates NMDA receptors
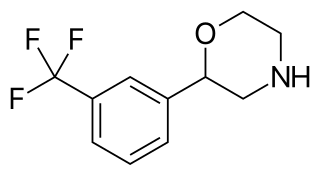
Flumexadol (INN) is a drug described and researched as a non-opioid analgesic which was never marketed. It has been found to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. According to Nilsson (2006) in a paper on 5-HT2C receptor agonists as potential anorectics, "The (+)-enantiomer of this compound showed [...] affinity for the 5-HT2C receptor (Ki) 25 nM) [...] and was 40-fold selective over the 5-HT2A receptor in receptor binding studies. Curiously, the racemic version [...], also known as 1841 CERM, was originally reported to possess analgesic properties while no association with 5-HT2C receptor activity was mentioned." It is implied that flumexadol might be employable as an anorectic in addition to analgesic. Though flumexadol itself has never been approved for medical use, oxaflozane is a prodrug of the compound that was formerly used clinically in France as an antidepressant and anxiolytic agent.

Lanepitant (INN, code name LY303870) is a drug developed by Eli Lilly which acts as a selective antagonist for the NK1 receptor, and was one of the first compounds developed that act at this target. It was under development as a potential analgesic drug, but despite promising results in initial animal studies, human clinical trials against migraine, arthritis and diabetic neuropathy all failed to show sufficient efficacy to support further development, with the drug being only marginally more effective than placebo and inferior to older comparison drugs such as naproxen. Failure of analgesic action was thought to be due to poor penetration of the blood–brain barrier in humans, but research has continued into potential applications in the treatment of other disorders with a peripheral site of action, such as corneal neovascularization.
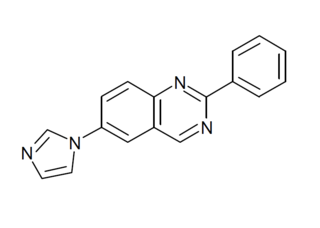
CR-4056 is an analgesic drug candidate with a novel mechanism of action, acting as a ligand for the imidazoline receptor I2. It showed promising results in animal studies against various types of neuropathic pain, and has reached Phase II human clinical trials as a potential treatment for pain associated with osteoarthritis.
















