The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.

Chlorphenamine, also known as chlorpheniramine, is an antihistamine used to treat the symptoms of allergic conditions such as allergic rhinitis. It is taken orally. The medication takes effect within two hours and lasts for about 4–6 hours. It is a first-generation antihistamine and works by blocking the H1 receptor.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.

Vanoxerine is an investigational drug which is being evaluated for the treatment of heart arrhythmias and cocaine dependence. Vanoxerine is a piperazine derivative which has multiple pharmacological activities including acting as an dopamine reuptake inhibitor, serotonin transporter inhibitor, and as a blocker of the cardiac hERG repolarizing potassium channel (IKr).

Oxatomide, sold under the brand name Tinset among others, is a antihistamine of the diphenylmethylpiperazine family which is marketed in Europe, Japan, and a number of other countries. It was discovered at Janssen Pharmaceutica in 1975. Oxatomide lacks any anticholinergic effects. In addition to its H1 receptor antagonism, it also possesses antiserotonergic activity similarly to hydroxyzine.

Antrafenine (Stakane) is a phenylpiperazine derivative drug invented in 1979. It acts as an analgesic and anti-inflammatory drug with similar efficacy to naproxen, but is not widely used as it has largely been replaced by newer drugs.
Fipexide is a psychoactive drug of the piperazine chemical class which was developed in Italy in 1983. It was used as a nootropic drug in Italy and France, mainly for the treatment of senile dementia, but is no longer in common use due to the occurrence of rare adverse drug reactions including fever and hepatitis. Fipexide is similar in action to other nootropic drugs such as piracetam and has a few similarities in chemical structure to centrophenoxine. Chemically, it is an amide union of parachlorophenoxyacetate and methylenedioxybenzylpiperazine (MDBZP), and has been shown to metabolize to the latter, which plays a significant role in its effects.
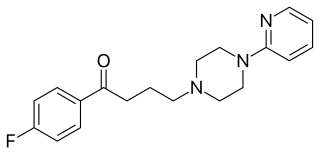
Azaperone is a pyridinylpiperazine and butyrophenone neuroleptic drug with sedative and antiemetic effects, which is used mainly as a tranquilizer in veterinary medicine. It is uncommonly used in humans as an antipsychotic drug.

Tandospirone, sold under the brand name Sediel, is an anxiolytic and antidepressant medication used in Japan and China, where it is marketed by Dainippon Sumitomo Pharma. It is a member of the azapirone class of drugs and is closely related to other azapirones like buspirone and gepirone.

Manidipine is a calcium channel blocker that is used clinically as an antihypertensive.
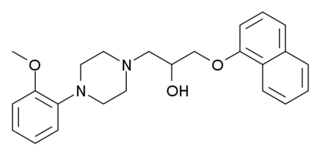
Naftopidil is a drug used in benign prostatic hypertrophy which acts as a selective α1-adrenergic receptor antagonist or alpha-1 blocker.

Clemizole is a histamine H1 receptor antagonist.

Atevirdine is a non-nucleoside reverse transcriptase inhibitor that has been studied for the treatment of HIV.

Dixyrazine, also known as dixypazin (oxalate), sold under the brand names Ansiolene, Esocalm, Esucos, Metronal, and Roscal, is a typical antipsychotic of the phenothiazine group described as a neuroleptic and antihistamine. It was first introduced in Germany in 1969. It is used as a neuroleptic, anxiolytic, and antihistamine in doses between 12.5 and 75 mg a day.

Thiopropazate is a typical antipsychotic of the phenothiazine class. It is a prodrug to perphenazine.

Adatanserin is a mixed 5-HT1A receptor partial agonist and 5-HT2A and 5-HT2C receptor antagonist. It was under development by Wyeth as an antidepressant but was ultimately not pursued.

Mazapertine (RWJ-37796) is an antipsychotic agent that was developed by Johnson & Johnson but never marketed. It exerts its pharmacological effect through affinity for dopamine D2, serotonin 5-HT1A, and α1-adrenergic receptors.
2,5-Diketopiperazine is an organic compound with the formula (NHCH2C(O))2. The compound features a six-membered ring containing two amide groups at opposite positions in the ring. It was first compound containing a peptide bond to be characterized by X-ray crystallography in 1938. It is the parent of a large class of 2,5-Diketopiperazines (2,5-DKPs) with the formula (NHCH2(R)C(O))2 (R = H, CH3, etc.). They are ubiquitous peptide in nature. They are often found in fermentation broths and yeast cultures as well as embedded in larger more complex architectures in a variety of natural products as well as several drugs. In addition, they are often produced as degradation products of polypeptides, especially in processed foods and beverages. They have also been identified in the contents of comets.

Diphenpipenol is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl)piperazine derivative related to compounds such as MT-45 and AD-1211, but diphenpipenol is the most potent compound in the series, with the more active (S) enantiomer being around 105 times the potency of morphine in animal studies. This makes it a similar strength to fentanyl and its analogues, and consequently diphenpipenol can be expected to pose a significant risk of producing life-threatening respiratory depression, as well as other typical opioid side effects such as sedation, itching, nausea and vomiting.


















