
Hydrocodone, also known as dihydrocodeinone, is a semi-synthetic opioid used to treat pain and as a cough suppressant. It is taken by mouth. Typically, it is dispensed as the combination acetaminophen/hydrocodone or ibuprofen/hydrocodone for pain severe enough to require an opioid and in combination with homatropine methylbromide to relieve cough. It is also available by itself in a long-acting form sold under the brand name Zohydro ER, among others, to treat severe pain of a prolonged duration. Hydrocodone is a controlled drug: in the United States, it is classified as a Schedule II Controlled Substance.

Morphine, formerly also called morphia, is an opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies. It is mainly used as an analgesic. There are numerous methods used to administer morphine: orally; administered under the tongue; via inhalation; injection into a vein, injection into a muscle, injection under the skin, or injection into the spinal cord area; transdermal; or via administered into the rectal canal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mouth, while the duration of its effect is 3–7 hours. Long-acting formulations of morphine are sold under the brand names MS Contin and Kadian, among others. Generic long-acting formulations are also available.
ATC code N02Analgesics is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup N02 is part of the anatomical group N Nervous system.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.
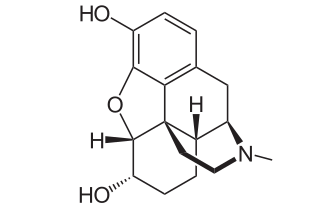
Dihydromorphine is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.

Etonitazene, also known as EA-4941 or CS-4640, is a benzimidazole opioid, first reported in 1957, that has been shown to have approximately 1,000 to 1,500 times the potency of morphine in animals.

Thebacon, or dihydrocodeinone enol acetate, is a semisynthetic opioid that is similar to hydrocodone and is most commonly synthesised from thebaine. Thebacon was invented in Germany in 1924, four years after the first synthesis of hydrocodone. Thebacon is a derivative of acetyldihydrocodeine, where only the 6–7 double bond is saturated. Thebacon is marketed as its hydrochloride salt under the trade name Acedicon, and as its bitartrate under Diacodin and other trade names. The hydrochloride salt has a free base conversion ratio of 0.846. Other salts used in research and other settings include thebacon's phosphate, hydrobromide, citrate, hydroiodide, and sulfate.

Nicomorphine is the 3,6-dinicotinate ester of morphine. It is a strong opioid agonist analgesic two to three times as potent as morphine with a side effect profile similar to that of dihydromorphine, morphine, and diamorphine.

Nicodicodine is an opioid developed as a cough suppressant and analgesic. Synthesized in 1904, it is not commonly used, but has activity similar to other opioids. Nicodicodine is metabolised in the liver by demethylation to produce 6-nicotinoyldihydromorphine, and subsequently further metabolised to dihydromorphine. Since the final active metabolite is the slightly stronger opiate dihydromorphine rather than morphine, nicodicodine can be expected to be marginally more potent and longer acting than nicocodeine. Side effects are similar to those of other opioids and include itching, nausea and respiratory depression.

Acetyldihydrocodeine is an opiate derivative discovered in Germany in 1914 and was used as a cough suppressant and analgesic. It is not commonly used, but has activity similar to other opiates. Acetyldihydrocodeine is a very close relative derivative of thebacon, where only the 6-7 bond is unsaturated. Acetyldihydrocodeine can be described as the 6-acetyl derivative of dihydrocodeine and is metabolised in the liver by demethylation and deacetylation to produce dihydromorphine.

Diacetyldihydromorphine is a potent opiate derivative developed in Germany in 1928 which is rarely used in some countries for the treatment of severe pain such as that caused by terminal cancer, as another form of diacetylmorphine. Diacetyldihydromorphine is fast-acting and longer-lasting than diamorphine, with a duration of action of around 4–7 hours.
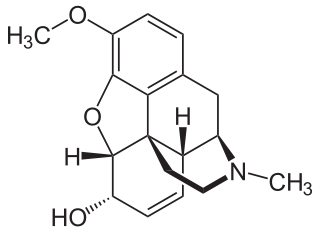
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children. In Europe, it is not recommended as a cough medicine for those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of addiction and overdose.

Benzylmorphine (Peronine) is a semi-synthetic opioid narcotic introduced to the international market in 1896 and that of the United States very shortly thereafter. It is much like codeine, containing a benzyl group attached to the morphine molecule just as the methyl group creates codeine and the ethyl group creates ethylmorphine or dionine. It is about 90% as strong as codeine by weight.

Nalorphine is a mixed opioid agonist–antagonist with opioid antagonist and analgesic properties. It was introduced in 1954 and was used as an antidote to reverse opioid overdose and in a challenge test to determine opioid dependence.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.

Codeine methylbromide (Eucodin) is the bromomethane (methylbromide) salt of codeine. Its possession is prohibited in many jurisdictions. It is considered a Schedule I controlled substance in the United States, with a DEA ACSCN of 9070 and nil annual aggregate manufacturing quota. as of 2014. As it is used in a different way than basic salts of codeine like the phosphate or hydrochloride owing to its below-mentioned dual action, it is considered to be a different drug related to codeine rather than merely a salt of it in many contexts.

An opiate is an alkaloid substance derived from opium. It differs from the similar term opioid in that the latter is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions, with evidence of opiate trade and use for pain relief as early as the eighth century AD. Most opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.

Acetylpropionylmorphine is an opiate analog that is an ester of morphine. It was developed in the early 1900s after first being synthesized in Great Britain in 1875 but shelved along with heroin and various other esters of morphine. Acetylpropionylmorphone was never used medically, instead being widely sold as one of the first "designer drugs" for around five years following the introduction of the first international restrictions on the sale of heroin in 1925. It is described as being virtually identical to heroin and morphine in its effects, and consequently was itself banned internationally in 1930 by the Health Committee of the League of Nations, in order to prevent its sale as an unscheduled alternative to heroin.

Morphine-N-oxide (genomorphine) is an active opioid metabolite of morphine. Morphine itself, in trials with rats, is 11–22 times more potent than morphine-N-oxide subcutaneously and 39–89 times more potent intraperitoneally. However, pretreatment with amiphenazole or tacrine increases the potency of morphine-N-oxide in relation to morphine. A possible explanation is that morphine-N-oxide is rapidly inactivated in the liver and impairment of inactivation processes or enzymes increases functionality.

Dibutyrylmorphine is the 3,6-dibutyryl ester of morphine, first synthesized by the CR Alders Wright organization in the United Kingdom in 1875.




















