
Dextromethorphan, or DXM, a common active ingredient found in many over-the-counter cough suppressant cold medicines, is used as a recreational drug and entheogen for its dissociative effects. Street names include Brownies, Dextro, Drix, Gel, Groove, Lean, Mega-perls, Poor man's ecstasy, Poor man's PCP, Red devils, Robo, Rojo, Rome, Skittles, Sizzurp, Sky and Velvet.

Cold medicines are a group of medications taken individually or in combination as a treatment for the symptoms of the common cold and similar conditions of the upper respiratory tract. The term encompasses a broad array of drugs, including analgesics, antihistamines and decongestants, among many others. It also includes drugs which are marketed as cough suppressants or antitussives, but their effectiveness in reducing cough symptoms is unclear or minimal.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.

Dextrorphan (DXO) is a psychoactive drug of the morphinan class which acts as an antitussive or cough suppressant and in high doses a dissociative hallucinogen. It is the dextrorotatory enantiomer of racemorphan; the levorotatory enantiomer is levorphanol. Dextrorphan is produced by O-demethylation of dextromethorphan by CYP2D6. Dextrorphan is an NMDA antagonist and contributes to the psychoactive effects of dextromethorphan.

Noscapine is a benzylisoquinoline alkaloid, of the phthalideisoquinoline structural subgroup, which has been isolated from numerous species of the family Papaveraceae. It lacks significant hypnotic, euphoric, or analgesic effects affording it with very low addictive potential. This agent is primarily used for its antitussive (cough-suppressing) effects.

Phenyltoloxamine is an antihistamine with sedative and analgesic effects. It is available in combination with other drugs such as paracetamol (acetominophen).

Dextromethorphan (DXM), sold under the trade name Robitussin among others, is a cough suppressant used in many cough and cold medicines. It affects serotonin, norepinephrine, NMDA, and sigma-1 receptors in the brain, all of which have been implicated in the pathophysiology of depression. In 2022, the FDA approved the combination dextromethorphan/bupropion to serve as a rapid acting antidepressant in patients with major depressive disorder.

Lefetamine (Santenol) is a drug which is a stimulant and also an analgesic with effects comparable to codeine.
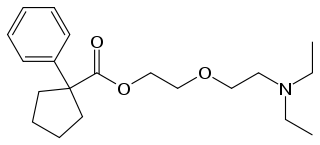
Pentoxyverine (rINN) or carbetapentane is an antitussive commonly used for cough associated with illnesses like common cold. It is sold over-the-counter as Solotuss, or in combination with other medications, especially decongestants. One such product is Certuss, a combination of guaifenesin and pentoxyverine. The drug has been available in the form of drops, suspensions and suppositories.

Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children. In Europe, it is not recommended as a cough medicine in those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of habituation and overdose.
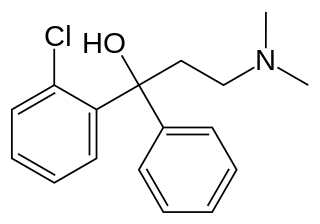
Clofedanol (INN) or chlophedianol (BAN), sold under the brand name Ninjacof among others, is a centrally acting cough suppressant used in the treatment of dry cough. Clofedanol has local anesthetic, antispasmodic, and antihistamine properties, and may have anticholinergic effects at high doses.

Dimethoxanate is a cough suppressant of the phenothiazine class.

Tipepidine (INN), also known as tipepidine hibenzate (JAN), is a synthetic, non-opioid antitussive and expectorant of the thiambutene class. It acts as an inhibitor of G protein-coupled inwardly-rectifying potassium channels (GIRKs). The drug was discovered in the 1950s, and was developed in Japan in 1959. It is used as the hibenzate and citrate salts.

Cloperastine (INN) or cloperastin, in the forms of cloperastine hydrochloride (JAN) and cloperastine fendizoate, is an antitussive and antihistamine that is marketed as a cough suppressant in Japan, Hong Kong, and in some European countries. It was first introduced in 1972 in Japan, and then in Italy in 1981.

Dibunate is a cough suppressant. As the sodium salt, it has been marketed under the name Becantyl, Becantex, or Linctussal with a dosage of 20 to 30 mg, as either syrup or tablets.

Zipeprol is a centrally acting cough suppressant developed in France in the 1970s. It is not a morphinan derivative. Zipeprol acts as a local anaesthetic and has mucolytic, antihistamine and anticholinergic properties. It is sold with several brand names such as Zinolta and Respilene. It is not available in the United States or Canada and has been discontinued in Europe. It is still available in some countries in Asia and South America.

Butamirate is a cough suppressant. It has been marketed in Europe and Mexico, but not in the United States.

Oxeladin is a cough suppressant. It is a highly potent and effective drug used to treat all types of cough of various etiologies. It is not related to opium or its derivatives, so treatment with oxeladin is free of risk of dependence or addiction. Oxeladin has none of the side effects which are present when common antitussives, such as codeine and its derivatives, are used. It may be used at every age, as well as in patients with heart disease, since it has a high level of safety and a great selectivity to act on the bulbar centre of cough.

Dimemorfan (INN), or dimemorfan phosphate (JAN), also known as 3,17-dimethylmorphinan, is an antitussive of the morphinan family that is widely used in Japan and is also marketed in Spain and Italy. It was developed by Yamanouchi Pharmaceutical and introduced in Japan in 1975. It was later introduced in Spain in 1981 and Japan in 1985.

An opiate is an alkaloid substance derived from opium. It differs from the similar term opioid in that the latter is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions, with evidence of opiate trade and use for pain relief as early as the eighth century AD. Most opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.




















