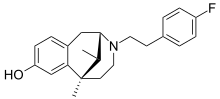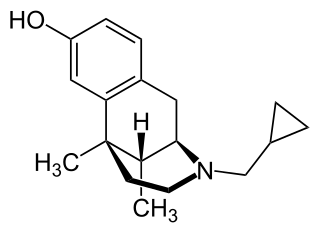
Cyclazocine is a mixed opioid agonist/antagonist related to dezocine, pentazocine and phenazocine. This family of opioid drugs is called the benzomorphans or benzazocines. It is a KOR agonist and MOR partial agonist, and also has high affinity for the DOR.

Ketazocine (INN), also known as ketocyclazocine, is a benzomorphan derivative used in opioid receptor research. Ketazocine, for which the receptor is named, is an exogenous opioid that binds to the κ opioid receptor.
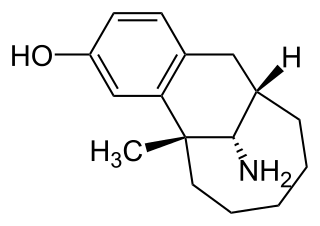
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.
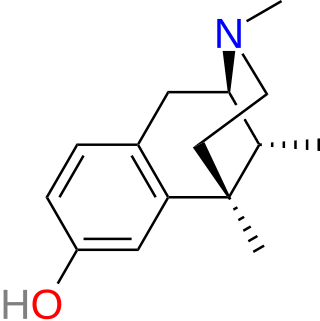
Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects, mediated through a mixed agonist–antagonist action at the mu opioid receptor, its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors and/or sigma receptors.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.

Bremazocine is a κ-opioid receptor agonist related to pentazocine. It has potent and long-lasting analgesic and diuretic effects. It has 200 times the activity of morphine, but appears to have no addictive properties and does not depress breathing. The crystal structure of bremazocine was determined in 1984

Benzomorphan is a chemical compound that is the base for a series of drugs which variably act on the sigma receptors and κ-opioid receptors, including the following compounds:

Dextrallorphan (DXA) is a chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist. It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter. As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.
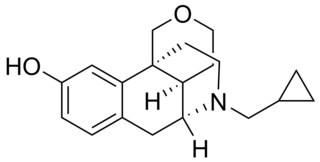
Proxorphan (INN), also known as proxorphan tartate (USAN), is an opioid analgesic and antitussive drug of the morphinan family that was never marketed. It acts preferentially as a κ-opioid receptor partial agonist and to a lesser extent as a μ-opioid receptor partial agonist.

8-Carboxamidocyclazocine (8-CAC) is an opioid analgesic drug related to cyclazocine, discovered by medicinal chemist Mark P. Wentland and co-workers in Cogswell Laboratory at Rensselaer Polytechnic Institute. Similarly to cyclazocine, 8-CAC acts as an agonist at both the μ- and κ-opioid receptors, but has a much longer duration of action than cyclazocine, and does not have μ antagonist activity. Unexpectedly, it was discovered that the phenolic hydroxyl group of cyclazocine could be replaced by a carboxamido group with only slight loss of potency at opioid receptors, and this discovery has subsequently been used to develop many novel opioid derivatives where the phenolic hydroxy group has been replaced by either carboxamide or a variety of larger groups. Due to their strong κ-opioid agonist activity, these drugs are not suited for use as analgesics in humans, but have instead been researched as potential drugs for the treatment of cocaine addiction.
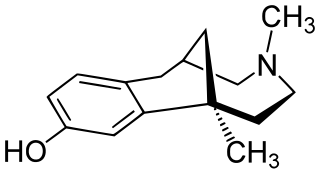
Eptazocine (Sedapain) is an opioid analgesic which was introduced in Japan by Morishita in 1987. It acts as a mixed κ-opioid receptor agonist and μ-opioid receptor antagonist.

Tonazocine (WIN-42,156) is an opioid analgesic of the benzomorphan family which made it to phase II clinical trials for the treatment of postoperative pain, but development was apparently ceased and ultimately it was never marketed. Tonazocine is a partial agonist at both the mu-opioid and delta-opioid receptors, but acting more like an antagonist at the former and more like an agonist at the latter. It lacks most of the side effects of other opioids such as adverse effects on the cardiovascular system and respiratory depression, but it can cause sedation, and in some patients it may induce hallucinations.
Deltorphin, also known as deltorphin A and dermenkephalin, is a naturally occurring, exogenous opioid heptapeptide and thus, exorphin, with the amino acid sequence Tyr-D-Met-Phe-His-Leu-Met-Asp-NH2. Along with the other deltorphins (such as deltorphin I and deltorphin II) and the dermorphins, deltorphin is endogenous to frogs of the genus Phyllomedusa such as P. bicolor and P. sauvagei where it is produced in their skin, and is not known to occur naturally in any other species. Deltorphin is one of the highest affinity and most selective naturally occurring opioid peptides known, acting as a very potent and highly specific agonist of the δ-opioid receptor.

Zenazocine is an opioid analgesic of the benzomorphan family which made it to phase II clinical trials before development was ultimately halted and it was never marketed. It acts as a partial agonist of the μ- and δ-opioid receptors, with less intrinsic activity at the former receptor and more at the latter receptor, and produces antinociceptive effects in animal studies.

Moxazocine (BL-4566) is an opioid analgesic of the benzomorphan family which was never marketed. It acts as a partial agonist or mixed agonist/antagonist of the opioid receptors and binds preferentially to the κ-opioid receptor. Despite its failure to reach the market, clinical studies demonstrated moxazocine to be approximately 10x as potent by weight as morphine as an analgesic.

Ethylketazocine (WIN-35,197-2), is an opioid drug of the benzomorphan family which has been used extensively in scientific research in the last few decades as a tool to aid in the study of the κ-opioid receptor. However, due to its relatively poor selectivity for the κ-opioid receptor over the μ- and δ-opioid receptors, as well as its relatively poor intrinsic activity at all sites, it has been mostly replaced in recent times by newer and more potent and selective compounds like U-50,488 and ICI-199,441.

Gemazocine (R-15,497), also known as cyclogemine, is a non-selective opioid antagonist of the benzomorphan class. It may have partial agonist properties at some of the opioid receptors, such as at the kappa receptor, but seems to be generally antagonistic in its actions.

Quadazocine (WIN-44,441) is an opioid antagonist of the benzomorphan family which is used in scientific research. It acts as a silent antagonist at all three of the major opioid receptors—μ, κ, and δ, but with a significant preference in affinity for the μ receptor and the κ2 subtype. As such, it has been touted as a "κ2-selective" antagonist, though this is not entirely accurate on account of its similar affinity for the μ receptor. As would be expected, quadazocine reverses the effects of opioid agonists like morphine and fentanyl in animals.
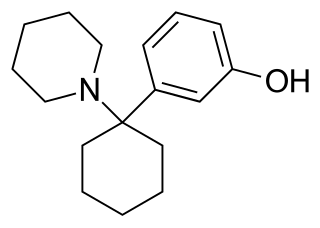
3-Hydroxyphencyclidine (3-HO-PCP) is a dissociative of the arylcyclohexylamine class related to phencyclidine (PCP) that has been sold online as a designer drug.