Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body.
Radionuclide therapy uses radioactive substances called radiopharmaceuticals to treat medical conditions, particularly cancer. These are introduced into the body by various means and localise to specific locations, organs or tissues depending on their properties and administration routes. This includes anything from a simple compound such as sodium iodide that locates to the thyroid via trapping the iodide ion, to complex biopharmaceuticals such as recombinant antibodies which are attached to radionuclides and seek out specific antigens on cell surfaces.
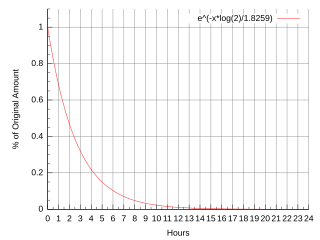
Fluorine-18 (18F) is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380(6) u and its half-life is 109.771(20) minutes. It decays by positron emission 96.7% of the time and electron capture 3.3% of the time. Both modes of decay yield stable oxygen-18.
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET).

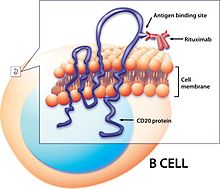
TAH molecule, also known as N-acetyl-L-aspartyl-L-glutamate peptidase I, NAAG peptidase, or prostate-specific membrane antigen (PSMA) is an enzyme that in humans is encoded by the FOLH1 gene. Human GCPII contains 750 amino acids and weighs approximately 84 kDa.

The sigma-2 receptor (σ2R) is a sigma receptor subtype that has attracted attention due to its involvement in diseases such as neurological diseases, neurodegenerative, neuro-ophthalmic and cancer. It is currently under investigation for its potential diagnostic and therapeutic uses.
Copper-64 (64Cu) is a positron and beta emitting isotope of copper, with applications for molecular radiotherapy and positron emission tomography. Its unusually long half-life (12.7-hours) for a positron-emitting isotope makes it increasingly useful when attached to various ligands, for PET and PET-CT scanning.
Indium-111 (111In) is a radioactive isotope of indium (In). It decays by electron capture to stable cadmium-111 with a half-life of 2.8 days. Indium-111 chloride (111InCl) solution is produced by proton irradiation of a cadmium target in a cyclotron, as recommended by International Atomic Energy Agency (IAEA). The former method is more commonly used as it results in a high level of radionuclide purity.
Preclinical imaging is the visualization of living animals for research purposes, such as drug development. Imaging modalities have long been crucial to the researcher in observing changes, either at the organ, tissue, cell, or molecular level, in animals responding to physiological or environmental changes. Imaging modalities that are non-invasive and in vivo have become especially important to study animal models longitudinally. Broadly speaking, these imaging systems can be categorized into primarily morphological/anatomical and primarily molecular imaging techniques. Techniques such as high-frequency micro-ultrasound, magnetic resonance imaging (MRI) and computed tomography (CT) are usually used for anatomical imaging, while optical imaging, positron emission tomography (PET), and single photon emission computed tomography (SPECT) are usually used for molecular visualizations.

DOTA-TATE is an eight amino acid long peptide, with a covalently bonded DOTA bifunctional chelator.
Advanced Accelerator Applications is a France-based pharmaceutical group, specialized in the field of nuclear medicine. The group operates in all three segments of nuclear medicine to diagnose and treat serious conditions in the fields of oncology, neurology, cardiology, infectious and inflammatory diseases.
A CXCR4 antagonist is a substance which blocks the CXCR4 receptor and prevent its activation. Blocking the receptor stops the receptor's ligand, CXCL12, from binding which prevents downstream effects. CXCR4 antagonists are especially important for hindering cancer progression because one of the downstream effects initiated by CXCR4 receptor activation is cell movement which helps the spread of cancer, known as metastasis. The CXCR4 receptor has been targeted by antagonistic substances since being identified as a co-receptor in HIV and assisting the development of cancer. Macrocyclic ligands have been utilised as CXCR4 antagonists.
A PSMA scan is a nuclear medicine imaging technique used in the diagnosis and staging of prostate cancer. It is carried out by injection of a radiopharmaceutical with a positron or gamma emitting radionuclide and a prostate-specific membrane antigen (PSMA) targeting ligand. After injection, imaging of positron emitters such as gallium-68 (68Ga), copper-64 (64Cu), and fluorine-18 (18F) is carried out with a positron emission tomography (PET) scanner. For gamma emitters such as technetium-99m (99mTc) and indium-111 (111In) single-photon emission computed tomography (SPECT) imaging is performed with a gamma camera.

Peptide receptor radionuclide therapy (PRRT) is a type of radionuclide therapy, using a radiopharmaceutical that targets peptide receptors to deliver localised treatment, typically for neuroendocrine tumours (NETs).

Lutetium (177Lu) oxodotreotide (INN) or 177Lu dotatate, brand name Lutathera, is a chelated complex of a radioisotope of the element lutetium with dotatate, used in peptide receptor radionuclide therapy. Specifically, it is used in the treatment of cancers which express somatostatin receptors. It is a radiolabeled somatostatin analog.

Gallium (68Ga) gozetotide or Gallium (68Ga) PSMA-11 sold under the brand name Illuccix among others, is a radiopharmaceutical made of 68Ga conjugated to prostate-specific membrane antigen (PSMA) targeting ligand, Glu-Urea-Lys(Ahx)-HBED-CC, used for imaging prostate cancer by positron emission tomography (PET). The PSMA targeting ligand specifically directs the radiolabeled imaging agent towards the prostate cancerous lesions in men.

Lutetium (177Lu) vipivotide tetraxetan, sold under the brand name Pluvicto, is a radiopharmaceutical medication used for the treatment of prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC). Lutetium (177Lu) vipivotide tetraxetan is a targeted radioligand therapy.

Somatostatin receptor antagonists are a class of chemical compounds that work by imitating the structure of the neuropeptide somatostatin. The somatostatin receptors are G protein-coupled receptors. Somatostatin receptor subtypes in humans are sstr1, 2A, 2 B, 3, 4 and 5. While normally expressed in the gastrointestinal (GI) tract, pancreas, hypothalamus, and central nervous system (CNS), they are expressed in different types of tumours. The predominant subtype in cancer cells is the ssrt2 subtype, which is expressed in neuroblastomas, meningiomas, medulloblastomas, breast carcinomas, lymphomas, renal cell carcinomas, paragangliomas, small cell lung carcinomas and hepatocellular carcinomas.

Somatostatin receptor antagonists are a class of chemical compounds that work by imitating the structure of the neuropeptide somatostatin, which is an endogenous hormone found in the human body. The somatostatin receptors are G protein-coupled receptors. Somatostatin receptor subtypes in humans include sstr1, 2A, 2 B, 3, 4, and 5. While normally expressed in the gastrointestinal (GI) tract, pancreas, hypothalamus, and central nervous system (CNS), they are expressed in different types of tumours. The predominant subtype in cancer cells is the ssrt2 subtype, which is expressed in neuroblastomas, meningiomas, medulloblastomas, breast carcinomas, lymphomas, renal cell carcinomas, paragangliomas, small cell lung carcinomas, and hepatocellular carcinomas.
Theranostics, also known as theragnostics, is a technique commonly used in personalised medicine. For example in nuclear medicine, one radioactive drug is used to identify (diagnose) and a second radioactive drug is used to treat (therapy) cancerous tumors. In other words, theranostics combines radionuclide imaging and radiation therapy which targets specific biological pathways.