Endorphins are peptides produced in the brain that block the perception of pain and increase feelings of wellbeing. They are produced and stored in the pituitary gland of the brain. Endorphins are endogenous painkillers often produced in the brain and adrenal medulla during physical exercise or orgasm and inhibit pain, muscle cramps, and relieve stress.

β-Endorphin (beta-endorphin) is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system. It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.
Dermorphin is a hepta-peptide first isolated from the skin of South American frogs belonging to the genus Phyllomedusa. The peptide is a natural opioid that binds as an agonist with high potency and selectivity to mu opioid receptors. Dermorphin is about 30–40 times more potent than morphine, but theoretically may be less likely to produce drug tolerance and addiction due to its high potency. The amino acid sequence of dermorphin is H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2.

Opioid peptides or opiate peptides are peptides that bind to opioid receptors in the brain; opiates and opioids mimic the effect of these peptides. Such peptides may be produced by the body itself, for example endorphins. The effects of these peptides vary, but they all resemble those of opiates. Brain opioid peptide systems are known to play an important role in motivation, emotion, attachment behaviour, the response to stress and pain, control of food intake, and the rewarding effects of alcohol and nicotine.

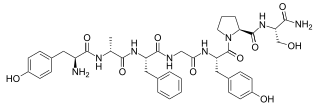
Endomorphins are considered to be natural opioid neuropeptides central to pain relief. The two known endomorphins, endomorphin-1 and endomorphin-2, are tetrapeptides, consisting of Tyr-Pro-Trp-Phe and Tyr-Pro-Phe-Phe amino acid sequences respectively. These sequences fold into tertiary structures with high specificity and affinity for the μ-opioid receptor, binding it exclusively and strongly. Bound μ-opioid receptors typically induce inhibitory effects on neuronal activity. Endomorphin-like immunoreactivity exists within the central and peripheral nervous systems, where endomorphin-1 appears to be concentrated in the brain and upper brainstem, and endomorphin-2 in the spinal cord and lower brainstem. Because endomorphins activate the μ-opioid receptor, which is the target receptor of morphine and its derivatives, endomorphins possess significant potential as analgesics with reduced side effects and risk of addiction.

Met-enkephalin, also known as metenkefalin (INN), sometimes referred to as opioid growth factor (OGF), is a naturally occurring, endogenous opioid peptide that has opioid effects of a relatively short duration. It is one of the two forms of enkephalin, the other being leu-enkephalin. The enkephalins are considered to be the primary endogenous ligands of the δ-opioid receptor, due to their high potency and selectivity for the site over the other endogenous opioids.

The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(mu)-opioid peptide (MOP) receptors. The prototypical μ-opioid receptor agonist is morphine, the primary psychoactive alkaloid in opium and for which the receptor was named, with mu being the first letter of Morpheus, the compound's namesake in the original Greek. It is an inhibitory G-protein coupled receptor that activates the Gi alpha subunit, inhibiting adenylate cyclase activity, lowering cAMP levels.

The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.
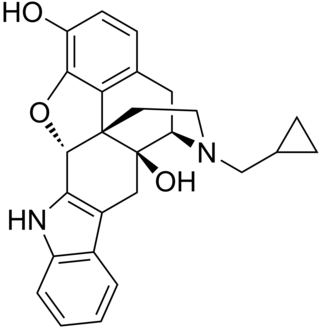
Naltrindole is a highly potent, highly selective delta opioid receptor antagonist used in biomedical research. In May 2012 a paper was published in Nature with the structure of naltrindole in complex with the mouse δ-opioid G-protein coupled receptor, solved by X-ray crystallography.

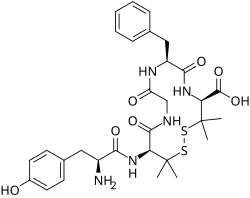
DADLE is a synthetic opioid peptide with analgesic properties. Although it is often considered a selective δ-opioid receptor agonist, it also binds to the μ1 subtype of μ-opioid receptors.
DAMGO is a synthetic opioid peptide with high μ-opioid receptor specificity. It was synthesized as a biologically stable analog of δ-opioid receptor-preferring endogenous opioids, leu- and met-enkephalin. Structures of DAMGO bound to the μ opioid receptor reveal a very similar binding pose to morphinans.

RB-101 is a drug that acts as an enkephalinase inhibitor, which is used in scientific research.
Neoendorphins are a group of endogenous opioid peptides derived from the proteolytic cleavage of prodynorphin. They include α-neoendorphin and β-neoendorphin. The α-neoendorphin is present in greater amounts in the brain than β-neoendorphin. Both are products of the dynorphin gene, which also expresses dynorphin A, dynorphin A-(1-8), and dynorphin B. These opioid neurotransmitters are especially active in Central Nervous System receptors, whose primary function is pain sensation. These peptides all have the consensus amino acid sequence of Try-Gly-Gly-Phe-Met (met-enkephalin) or Tyr-Gly-Gly-Phe-Leu ( leu-enkephalin). Binding of neoendorphins to opioid receptors (OPR), in the dorsal root ganglion (DRG) neurons results in the reduction of time of calcium-dependent action potential. The α-neoendorphins bind OPRD1(delta), OPRK1(kappa), and OPRM1 (mu) and β-neoendorphin bind OPRK1.
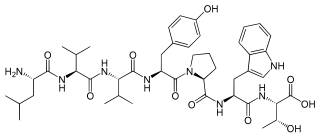
Spinorphin is an endogenous, non-classical opioid peptide of the hemorphin family first isolated from the bovine spinal cord (hence the prefix spin-) and acts as a regulator of the enkephalinases, a class of enzymes that break down endogenous the enkephalin peptides. It does so by inhibiting the enzymes aminopeptidase N (APN), dipeptidyl peptidase III (DPP3), angiotensin-converting enzyme (ACE), and neutral endopeptidase (NEP). Spinorphin is a heptapeptide and has the amino acid sequence Leu-Val-Val-Tyr-Pro-Trp-Thr (LVVYPWT). It has been observed to possess antinociceptive, antiallodynic, and anti-inflammatory properties. The mechanism of action of spinorphin has not been fully elucidated (i.e., how it acts to inhibit the enkephalinases), but it has been found to act as an antagonist of the P2X3 receptor, and as a weak partial agonist/antagonist of the FP1 receptor.

Hemorphin-4 is an endogenous opioid peptide of the hemorphin family which possesses antinociceptive properties and is derived from the β-chain of hemoglobin in the bloodstream. It is a tetrapeptide with the amino acid sequence Tyr-Pro-Trp-Thr. Hemorphin-4 has affinities for the μ-, δ-, and κ-opioid receptors that are in the same range as the structurally related β-casomorphins, although affinity to the κ-opioid receptor is markedly higher in comparison. It acts as an agonist at these sites. Hemorphin-4 also has inhibitory effects on angiotensin-converting enzyme (ACE), and as a result, may play a role in the regulation of blood pressure. Notably, inhibition of ACE also reduces enkephalin catabolism.

Biphalin is a dimeric enkephalin endogenous peptide (Tyr-D-Ala-Gly-Phe-NH)2 composed of two tetrapeptides derived from enkephalins, connected 'tail-to-tail' by a hydrazide bridge. The presence of two distinct pharmacophores confers on biphalin a high affinity for both μ and δ opioid receptors (with an EC50 of about 1–5 nM for both μ and δ receptors), therefore it has analgesic activity. Biphalin presents a considerable antinociceptive profile. In fact, when administered intracerebroventricularly in mice, biphalin displays a potency almost 7-fold greater than that of the ultra-potent alkaloid agonist, etorphine and 7000-fold greater than morphine; biphalin and morphine were found to be equipotent after intraperitoneal administration. The extraordinary in vivo potency shown by this compound is coupled with low side-effects, in particular, to produce no dependency in chronic use. For these reasons, several efforts have been carried out in order to obtain more information about structure-activity relationship (SAR). Results clearly indicate that, at least for μ receptor binding, the presence of two pharmacophores is not necessary; Tyr1 is indispensable for analgesic activity, while replacing Phe at the position 4 and 4' with non-aromatic, but lipophilic amino acids does not greatly change the binding properties and in general 4,4' positions are found to be important to design biphalin analogues with increased potency and modified μ/δ selectivity. The hydrazide linker is not fundamental for activity or binding, and it can be conveniently substituted by different conformationally constrained cycloaliphatic diamine linkers.
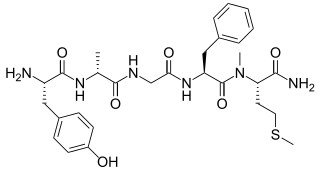
Metkefamide (INN; LY-127,623), or metkephamid acetate (USAN), but most frequently referred to simply as metkephamid, is a synthetic opioid pentapeptide and derivative of [Met]enkephalin with the amino acid sequence Tyr-D-Ala-Gly-Phe-(N-Me)-Met-NH2. It behaves as a potent agonist of the δ- and μ-opioid receptors with roughly equipotent affinity, and also has similarly high affinity as well as subtype-selectivity for the κ3-opioid receptor.

Endomorphin-2 (EM-2) is an endogenous opioid peptide and one of the two endomorphins. It has the amino acid sequence Tyr-Pro-Phe-Phe-NH2. It is a high affinity, highly selective agonist of the μ-opioid receptor, and along with endomorphin-1 (EM-1), has been proposed to be the actual endogenous ligand of this receptor (that is, rather than the endorphins). Like EM-1, EM-2 produces analgesia in animals, but whereas EM-1 is more prevalent in the brain, EM-2 is more prevalent in the spinal cord. In addition, the action of EM-2 differs from that of EM-1 somewhat, because EM-2 additionally induces the release of dynorphin A and [Met]enkephalin in the spinal cord and brain by an unknown mechanism, which in turn activate the κ- and δ-opioid receptors, respectively, and a portion of the analgesic effects of EM-2 is dependent on this action. Moreover, while EM-1 produces conditioned place preference, a measure of drug reward, EM-2 produces conditioned place aversion, an effect which is dynorphin A-dependent. Similarly to the case of EM-1, the gene encoding for EM-2 has not yet been identified.
John Hughes is a British neuroscientist who shared the 1978 Albert Lasker Award for Basic Medical Research for the discovery of met-enkephalin and leu-enkephalin. This discovery demonstrated that opiate drugs exert their effects on the human brain by mimicking endogenous neurotransmitters, the opioid peptides.

LIH383 is an octapeptide and highly potent and selective agonist of the atypical chemokine receptor ACKR3 (CXCR7) that was derived from the opioid peptide adrenorphin. ACKR3 is a novel opioid receptor which functions as a broad-spectrum trap or scavenger for endogenous opioid peptides, including enkephalins, dynorphins, and nociceptin, and thereby acts as a negative modulator of the opioid system. By displacing them from ACKR3 and thereby increasing their availability, LIH383 potentiates the actions of endogenous opioids, for instance their analgesic effects. Other ligands of ACKR3 include conolidine, CCX771, RTI-5152-12, and VUF15485.