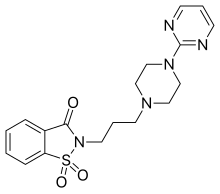
Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.

8-OH-DPAT is a research chemical of the aminotetralin chemical class which was developed in the 1980s and has been widely used to study the function of the 5-HT1A receptor. It was one of the first major 5-HT1A receptor full agonists to be discovered.

5-Carboxamidotryptamine (5-CT) is a tryptamine derivative closely related to the neurotransmitter serotonin.

Binospirone (MDL-73,005-EF) is a drug which acts as a partial agonist at 5-HT1A somatodendritic autoreceptors but as an antagonist at postsynaptic 5-HT1A receptors. It has anxiolytic effects.

YM-348 is an indazole derivative drug which acts as a potent and selective 5-HT2C receptor agonist, with an EC50 of 1nM and 15x selectivity over 5-HT2A, although it only has moderate selectivity of 3x over the closely related 5-HT2B receptor. It has thermogenic and anorectic effects in animal studies, making it potentially useful for the treatment of obesity.

CP-94253 is a drug which acts as a potent and selective serotonin 5-HT1B receptor agonist, with approximately 25x and 40x selectivity over the closely related 5-HT1D and 5-HT1A receptors. It has a range of behavioral effects, based on animal testing. The effects include the following: promoting wakefulness by increasing dopamine release in the brain; reducing food intake and promoting satiety; enhancing the reinforcing effects of cocaine; and possible antidepressant effects.A recent study found that "Regardless of sex, CP94253 decreased cocaine intake after abstinence and during resumption of SA [self-administration] and decreased cue reactivity" suggesting that agonism of the inhibitory 5-HT2B receptors may diminish the cognitive reward of cocaine usage and increased use of the drug without a period of abstinence may be a product of test subjects trying to achieve a previously rewarding experience through larger dosages of cocaine.

Ro60-0175 is a drug developed by Hoffmann–La Roche, which has applications in scientific research. It acts as a potent and selective agonist for both the 5-HT2B and 5-HT2C serotonin receptor subtypes, with good selectivity over the closely related 5-HT2A subtype, and little or no affinity at other receptors.

Zalospirone (WY-47,846) is a selective 5-HT1A partial agonist of the azapirone chemical class. It was found to be effective in the treatment of anxiety and depression in clinical trials, but a high proportion of subjects dropped out due to side effects and development was subsequently never completed.
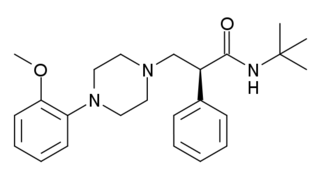
WAY-100135 is a serotonergic drug of the phenylpiperazine family which is used in scientific research. It acts as potent 5-HT1A receptor antagonist, and was originally believed to be highly selective, but further studies have demonstrated that it also acts as a partial agonist of the 5-HT1D receptor (pKi = 7.58; virtually the same affinity for 5-HT1A), and to a much lesser extent, of the 5-HT1B receptor (pKi = 5.82). These findings may have prompted the development of the related compound WAY-100635, another purportedly selective and even more potent 5-HT1A antagonist, which was synthesized shortly thereafter. However, WAY-100635 turned out to be non-selective as well, having been shown to act additionally as a potent D4 receptor agonist later on.

Alnespirone (S-20,499) is a selective 5-HT1A receptor full agonist of the azapirone chemical class. It has antidepressant and anxiolytic effects.

Eptapirone (F-11,440) is a very potent and highly selective 5-HT1A receptor full agonist of the azapirone family. Its affinity for the 5-HT1A receptor was reported to be 4.8 nM (Ki), and its intrinsic activity approximately equal to that of serotonin.
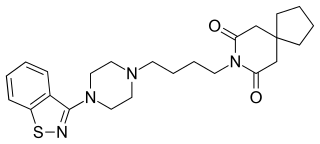
Tiospirone (BMY-13,859), also sometimes called tiaspirone or tiosperone, is an atypical antipsychotic of the azapirone class. It was investigated as a treatment for schizophrenia in the late 1980s and was found to have an effectiveness equivalent to those of typical antipsychotics in clinical trials but without causing extrapyramidal side effects. However, development was halted and it was not marketed. Perospirone, another azapirone derivative with antipsychotic properties, was synthesized and assayed several years after tiospirone. It was found to be both more potent and more selective in comparison and was commercialized instead.

Umespirone (KC-9172) is a drug of the azapirone class which possesses anxiolytic and antipsychotic properties. It behaves as a 5-HT1A receptor partial agonist (Ki = 15 nM), D2 receptor partial agonist (Ki = 23 nM), and α1-adrenoceptor receptor antagonist (Ki = 14 nM), and also has weak affinity for the sigma receptor (Ki = 558 nM). Unlike many other anxiolytics and antipsychotics, umespirone produces minimal sedation, cognitive/memory impairment, catalepsy, and extrapyramidal symptoms.

Lesopitron (E-4424) is a selective full agonist of the 5-HT1A receptor which is structurally related to the azapirones. In 2001 it was under development by Esteve as an anxiolytic for the treatment of generalized anxiety disorder (GAD). It made it to phase II clinical trials but was apparently discontinued as no new information on lesopitron has surfaced since.
Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor. Osemozotan has antidepressant, anxiolytic, antiobsessional, serenic, and analgesic effects in animal studies, and is used to investigate the role of 5-HT1A receptors in modulating the release of dopamine and serotonin in the brain, and their involvement in addiction to abused stimulants such as cocaine and methamphetamine.

S-14671 is a naphthylpiperazine derivative which acts as a 5-HT1A receptor agonist (pKi = 9.3) with high efficacy and exceptional in vivo potency, and also as a 5-HT2A and 5-HT2C receptor antagonist (both are pKi = 7.8). It displays only low and non-significant affinity for 5-HT1B and 5-HT3 sites.

Indorenate (TR-3369), is a tryptamine derivative which acts as an agonist at the 5-HT1A, 5-HT1B and 5-HT2C serotonin receptors. It has anxiolytic, antihypertensive and anorectic effects, predominantly through action at 5-HT1A, but with some contribution from the 5-HT1B and 5-HT2C subtypes, and possibly some other non-serotonergic targets also.

GR-113808 is a drug which acts as a potent and selective 5-HT4 serotonin receptor antagonist. It is used in researching the roles of 5-HT4 receptors in various processes, and has been used to test some of the proposed therapeutic effects of selective 5-HT4 agonists, such as for instance blocking the nootropic effects of 5-HT4 agonists, and worsening the respiratory depression produced by opioid analgesic drugs, which appears to be partly 5-HT4 mediated and can be counteracted by certain 5-HT4 agonists.

Bay R 1531 is a tricyclic tryptamine derivative which acts as a selective serotonin receptor 5-HT1A agonist. It was researched unsuccessfully for the treatment of stroke but remains in use for scientific research.