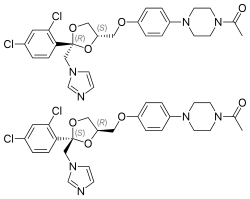
 (2R,4S)-(+)-ketoconazole (top) (2S,4R)-(−)-ketoconazole (bottom) | |
 Ball-and-stick model of (2R,4S)-(+)-ketoconazole | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌkiːtoʊˈkoʊnəˌzoʊl,-zɒl/ [1] [2] |
| Trade names | Nizoral, others |
| Other names | R-41400; KW-1414 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682816 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets), topical (cream, shampoo, solution) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | By mouth: 37–97% [7] |
| Protein binding | 84 to 99% |
| Metabolism | Extensive liver (predominantly oxidation, O-dealkylation) |
| Metabolites | N-deacetyl ketoconazole |
| Elimination half-life | Biphasic |
| Excretion | Bile duct (major) and kidney [8] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.059.680 |
| Chemical and physical data | |
| Formula | C26H28Cl2N4O4 |
| Molar mass | 531.43 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture [8] [9] |
| |
| |
| | |

Ketoconazole, sold under the brand name Nizoral, among others, is an antiandrogen, antifungal, and antiglucocorticoid medication used to treat a number of fungal infections. [10] Applied to the skin it is used for fungal skin infections such as tinea, cutaneous candidiasis, pityriasis versicolor, dandruff, and seborrheic dermatitis. [11] Taken by mouth it is a less preferred option and recommended for only severe infections when other agents cannot be used. [10] Other uses include treatment of excessive male-patterned hair growth in women and Cushing's syndrome. [10]
Contents
- Medical uses
- Topical antifungal
- Systemic antifungal
- Off-label uses
- Contraindications
- Side effects
- Gastrointestinal
- Endocrine
- Liver
- Hypersensitivity
- Topical formulations
- Pregnancy
- Overdose
- Interactions
- Pharmacology
- Pharmacodynamics
- Pharmacokinetics
- Chemistry
- History
- Society and culture
- Generic names
- Brand names
- Availability
- Research
- Veterinary use
- References
Common side effects when applied to the skin include redness. [11] Common side effects when taken by mouth include nausea, headache, and liver problems. [10] Liver problems may result in death or the need for a liver transplantation. [10] [12] Other severe side effects when taken orally include QT prolongation, adrenocortical insufficiency, and anaphylaxis. [10] [12] It is an imidazole and works by hindering the production of ergosterol required for the fungal cell membrane, thereby slowing growth. [10]
Ketoconazole was patented in 1977 by Belgian pharmaceutical company Janssen, and came into medical use in 1981. [13] It is available as a generic medication and formulations that are applied to the skin are over the counter in the United Kingdom. [11] In 2023, it was the 140th most commonly prescribed medication in the United States, with more than 3 million prescriptions. [14] [15] The formulation that is taken by mouth was withdrawn in the European Union and in Australia in 2013, [16] [17] and in China in 2015. [18] In addition, its use was restricted in the United States and Canada in 2013. [17]

