This article needs attention from an expert in chemicals. The specific problem is: chemical names need verification and correction as necessary.(September 2016) |
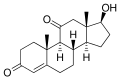
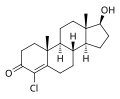
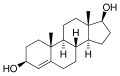
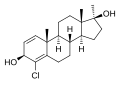
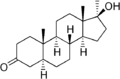
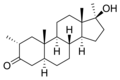
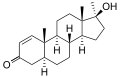
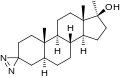
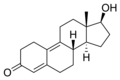
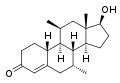
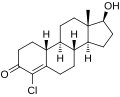
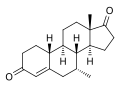
This is a list of androgens/anabolic steroids (AAS) or testosterone derivatives. Androgen esters are mostly not included in this list. The major classes of testosterone derivatives include the following (as well as combinations thereof):
Contents
- Natural/endogenous
- Testosterone derivatives
- Non-17α-Alkylated
- 17α-Alkylated
- 17α-alkenyl and -alkynyl
- Dihydrotestosterone derivatives
- Non-17α-Alkylated 2
- 17α-Alkylated 2
- 19-Nortestosterone (nandrolone) derivatives
- Non-17α-alkylated 3
- 17α-Alkylated 3
- 17α-alkenyl and -alkynyl 2
- See also
- Notes
- References
- Further reading
- Testosterone derivatives: direct derivatives of testosterone not falling into the groups below
- 4,5α-Reduced/dihydrogenated testosterone derivatives: dihydrotestosterone (DHT) derivatives
- 19-Demethylated testosterone derivatives: 19-nortestosterone (nandrolone) derivatives
- 17α-Alkylated testosterone derivatives: methyltestosterone and ethyltestosterone derivatives
- 17α-Ethynylated/vinylated testosterone derivatives: ethynyltestosterone (ethisterone) and vinyltestosterone derivatives
The last group consists of progestins with mostly only very weak androgenic/anabolic activity. AAS that are listed as marketed may be marketed as one or more esters rather than as the listed AAS itself.
This article pertains to steroidal androgens; nonsteroidal androgens like the selective androgen receptor modulators (SARMs) andarine and enobosarm (ostarine) are not included here.