
In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

Eli Lilly and Company, doing business as Lilly, is an American multinational pharmaceutical company headquartered in Indianapolis, Indiana, with offices in 18 countries. Its products are sold in approximately 125 countries. The company was founded in 1876 by Eli Lilly, a pharmaceutical chemist and Union Army veteran of the American Civil War for whom the company was later named.

Zosuquidar is an experimental antineoplastic drug. Zosquidir inhibits P-glycoproteins. Other drugs with this mechanism include tariquidar and laniquidar. P-glycoproteins are trans-membrane proteins that pump foreign substances out of cells in an ATP dependent fashion. Cancers overexpressing P-glycoproteins are able to pump out therapeutic molecules before they are able to reach their target, effectively making the cancer multi-drug resistant. Zosuquidar inhibits P-glycoproteins, inhibiting the efflux pump and restoring sensitivity to chemotherapeutic agents.
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.

Eli Lilly was a Union Army officer, pharmacist, chemist, and businessman who founded Eli Lilly and Company.
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2) at the 2-position with an oxygen atom. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals.

The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid, but usually either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds. Note that trihalomethyl ketone substrates will result in haloform and carboxylate formation via the haloform reaction instead.
The Blaise ketone synthesis is the chemical reaction of acid chlorides with organozinc compounds to give ketones.

Levopropoxyphene is an antitussive. It is an optical isomer of dextropropoxyphene. The racemic mixture is called propoxyphene. Only the dextro-isomer (dextropropoxyphene) has an analgesic effect; the levo-isomer appears to exert only an antitussive effect. It was formerly marketed in the U.S. by Eli Lilly under the tradename Novrad as an antitussive. Unlike many antitussives, it binds poorly to the sigma-1 receptor.

Crotonaldehyde is a chemical compound with the formula CH3CH=CHCHO. The compound is usually sold as a mixture of the E- and Z-isomers, which differ with respect to the relative position of the methyl and formyl groups. The E-isomer is more common (data given in Table is for the E-isomer). This lachrymatory liquid is moderately soluble in water and miscible in organic solvents. As an unsaturated aldehyde, crotonaldehyde is a versatile intermediate in organic synthesis. It occurs in a variety of foodstuffs, e.g. soybean oils.

The Stork enamine alkylation involves the addition of an enamine to a Michael acceptor or another electrophilic alkylation reagent to give an alkylated iminium product, which is hydrolyzed by dilute aqueous acid to give the alkylated ketone or aldehyde. Since enamines are generally produced from ketones or aldehydes, this overall process constitutes a selective monoalkylation of a ketone or aldehyde, a process that may be difficult to achieve directly.
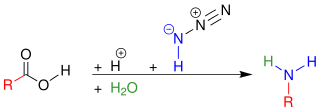
In organic chemistry, the Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophenone and hydrazoic acid to benzanilide. The intramolecular reaction was not reported until 1991 but has become important in the synthesis of natural products. The reaction is effective with carboxylic acids to give amines (above), and with ketones to give amides (below).

In chemistry, the haloform reaction is a chemical reaction in which a haloform is produced by the exhaustive halogenation of an acetyl group, in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce chloroform, bromoform, or iodoform. Note that fluoroform can't be prepared in this way.
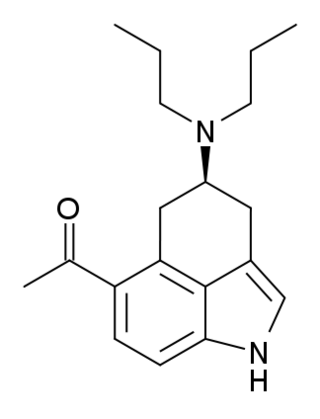
LY-293284 is a research chemical developed by the pharmaceutical company Eli Lilly and used for scientific studies. It acts as a potent and selective 5-HT1A receptor full agonist. It was derived through structural simplification of the ergoline based psychedelic LSD, but is far more selective for 5-HT1A with over 1000× selectivity over other serotonin receptor subtypes and other targets. It has anxiogenic effects in animal studies.
Alanna Schepartz is an American professor and scientist. She is currently the T.Z. and Irmgard Chu Distinguished Chair in Chemistry at University of California, Berkeley. She was formerly the Sterling Professor of Chemistry at Yale University.
The Eli Lilly Award in Biological Chemistry was established in 1934. Consisting of a bronze medal and honorarium, its purpose is to stimulate fundamental research in biological chemistry by scientists not over thirty-eight years of age. The Award is administered by the Division of Biological Chemistry of the American Chemical Society.
John C. Lechleiter is an American businessman and chemist. He served as president, chief executive officer, and chairman of the board of directors of Eli Lilly and Company from April 2008 to December 2016.
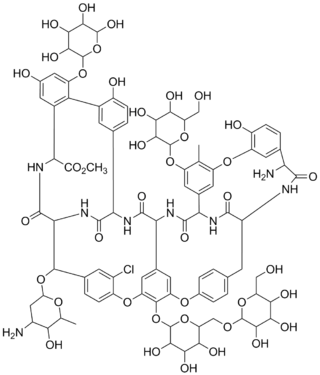
Actaplanin is a complex of broad-spectrum antibiotics made by Actinoplanes bacteria. Research carried out by a group in Eli Lilly and Co. in 1984 identified several actaplanins using high-performance liquid chromatography. Actaplanins A, B1, B2, B3, C1 and G were shown to be composed of the same peptide core, an amino sugar, and varying amounts of glucose, mannose, and rhamnose.
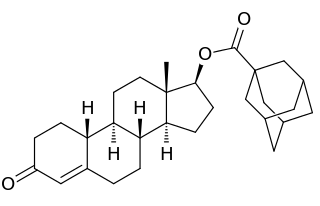
Bolmantalate, also known as 19-nortestosterone 17β-adamantoate, is an androgen and anabolic steroid and a nandrolone ester which was synthesized and developed by Eli Lilly in 1865 but was never marketed.

Flumezapine is an abandoned, investigational antipsychotic drug that was studied for the treatment of schizophrenia. Flumezapine failed clinical trials due to concern for liver and muscle toxicity. Flumezapine is structurally related to the common antipsychotic olanzapine—a point that was used against its manufacturer, Eli Lilly and Company, in a lawsuit in which generic manufacturers sought to void the patent on brand name olanzapine (Zyprexa). Although flumezapine does not differ greatly from olanzapine in terms of its structure, the difference was considered to be non-obvious, and Eli Lilly's patent rights on Zyprexa were upheld.















