 | |
| Clinical data | |
|---|---|
| Trade names | Winlevi |
| Other names | CB-03-01; Breezula; 11-Deoxycortisol 17α-propionate; 17α-(Propionyloxy)- deoxycorticosterone; 21-Hydroxy-3,20-dioxopregn-4-en-17-yl propionate |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.810 |
| Chemical and physical data | |
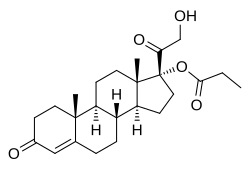
| Formula | C24H34O5 |
| Molar mass | 402.531 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clascoterone, sold under the brand name Winlevi, is an antiandrogen medication which is used topically in the treatment of acne. [5] The medication is used as a cream by application to the skin, for instance the face and scalp. [6] Clascoterone is an antiandrogen, or antagonist of the androgen receptor (AR), the biological target of androgens such as testosterone and dihydrotestosterone. [7] [8] It shows minimal systemic absorption when applied to skin. [6]
Contents
- Medical uses
- Side effects
- Pharmacology
- Pharmacodynamics
- Pharmacokinetics
- Chemistry
- History
- Society and culture
- Legal status
- Names
- Research
- References
- External links
Clascoterone was developed by Cassiopea and was approved for medical use in the United States in August 2020. [9] [10] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. [11]