
Ethylestrenol, also known as ethyloestrenol or ethylnandrol and sold under the brand names Maxibolin and Orabolin among others, is an androgen and anabolic steroid (AAS) medication which has been used in the past for a variety of indications such as to promote weight gain and to treat anemia and osteoporosis but has been discontinued for use in humans. It is still available for veterinary use in Australia and New Zealand however. It is taken by mouth.

Etynodiol diacetate, or ethynodiol diacetate, sold under the brand names Demulen and Femulen among others, is a progestin medication which is used in birth control pills. The medication is available only in combination with an estrogen. It is taken by mouth.

Metribolone is a synthetic and orally active anabolic–androgenic steroid (AAS) and a 17α-alkylated nandrolone (19-nortestosterone) derivative which was never marketed for medical use but has been widely used in scientific research as a hot ligand in androgen receptor (AR) ligand binding assays (LBAs) and as a photoaffinity label for the AR. More precisely, metribolone is the 17α-methylated derivative of trenbolone. It was investigated briefly for the treatment of advanced breast cancer in women in the late 1960s and early 1970s, but was found to produce signs of severe hepatotoxicity at very low dosages, and its development was subsequently discontinued.

Methandriol, also known as methylandrostenediol, is an androgen and anabolic steroid (AAS) medication which was developed by Organon and is used in both oral and injectable formulations. It is an orally active 17α-alkylated AAS and a derivative of the endogenous androgen prohormone androstenediol.

Bolenol, also known as 17α-ethyl-19-norandrost-5-en-17β-ol (ethylnorandrostenol), is a synthetic, orally active anabolic-androgenic steroid (AAS) and a 17α-alkylated derivative of 19-nortestosterone (nandrolone) that was never marketed. It was described in the literature in 1969.
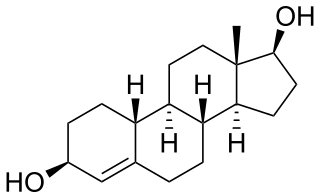
Bolandiol is an anabolic-androgenic steroid (AAS) that was never marketed. However, a dipropionate ester derivative, bolandiol dipropionate, has been marketed. Bolandiol and its dipropionate ester are unique among AASs in that they reportedly also possesses estrogenic and progestogenic activity.

Oxabolone is a synthetic anabolic-androgenic steroid (AAS) of the nandrolone (19-nortestosterone) group which was never marketed. It can be formulated as the cipionate ester prodrug oxabolone cipionate, which, in contrast, has been marketed for medical use.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

Bolandiol dipropionate (USAN), or bolandiol propionate (JAN), also known as norpropandrolate or 19-nor-4-androstenediol dipropionate, as well as estr-4-ene-3β,17β-diol 3,17-dipropionate, is a synthetic anabolic-androgenic steroid (AAS) and derivative of 19-nortestosterone (nandrolone). It is an androgen ester – specifically, the 3,17-dipropionate ester of bolandiol (19-nor-4-androstenediol).

Ethyltestosterone, or 17α-ethyltestosterone, also known as 17α-ethylandrost-4-en-17β-ol-3-one or 17α-pregn-4-en-17-ol-3-one, is a synthetic, orally active anabolic–androgenic steroid (AAS) of the 17α-alkylated group related to methyltestosterone which was never marketed. Like methyltestosterone, ethyltestosterone is the parent compound of many AAS. Derivatives of ethyltestosterone include norethandrolone, ethylestrenol (ethylnandrol), norboletone, ethyldienolone, tetrahydrogestrinone, bolenol (ethylnorandrostenol), and propetandrol.

Vinyltestosterone is a synthetic anabolic–androgenic steroid (AAS) that was never marketed. However, two 19-nortestosterone derivatives of vinyltestosterone, norvinisterone (17α-vinyl-19-nortestosterone) and norgesterone, have been marketed. They are used as progestins for female hormonal contraception, rather than as AAS.
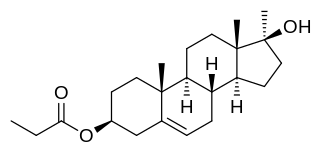
Methandriol propionate, or methylandrostenediol propionate, also known as 17α-methylandrost-5-ene-3β,17β-diol 3β-propionate, is a synthetic, injected anabolic-androgenic steroid (AAS) and a 17α-alkylated derivative of 5-androstenediol that is or was marketed by Vister in Italy. It is an androgen ester – specifically, the C3,17β propionate ester of methandriol (17α-methyl-5-androstenediol) – and acts as a prodrug of methandriol in the body. Methandriol propionate is administered by intramuscular injection and, relative to methandriol, has an extended duration via this route due to a depot effect afforded by its ester.
Methandriol bisenanthoyl acetate, or methylandrostenediol bisenanthoyl acetate, also known as 17α-methylandrost-5-ene-3β,17β-diol 3β,17β-di(3-oxononanoate), is a synthetic, injected anabolic–androgenic steroid (AAS) and a 17α-alkylated derivative of 5-androstenediol. It is an androgen ester—specifically, the C3β,17β di(3-oxononanoate) ester of methandriol (17α-methyl-5-androstenediol)—and acts as a prodrug of methandriol in the body. Methandriol bisenanthoyl acetate is administered by intramuscular injection and, relative to methandriol, has an extended duration via this route due to a depot effect afforded by its ester.
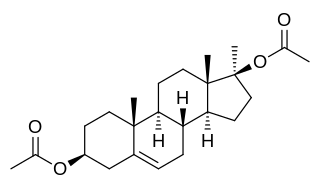
Methandriol diacetate, or methylandrostenediol diacetate, also known as 17α-methylandrost-5-ene-3β,17β-diol 3β,17β-diacetate, is a synthetic, injected anabolic–androgenic steroid (AAS) and a 17α-alkylated derivative of 5-androstenediol that was never marketed. It is an androgen ester – specifically, the C3,17β diacetate ester of methandriol (17α-methyl-5-androstenediol) – and acts as a prodrug of methandriol in the body.

6,6-Difluoronorethisterone, also known as 6,6-difluoro-17α-ethynyl-19-nortestosterone or as 6,6-difluoro-17α-ethynylestr-4-en-17β-ol-3-one, is a steroidal progestin of the 19-nortestosterone group that was described in 1971 but was never marketed. It is a fluorinated derivative of norethisterone. The C17β acetate ester, 6,6-difluoronorethisterone acetate, has also been synthesized and described.

6,6-Difluoronorethisterone acetate, also known as 6,6-difluoro-17α-ethynyl-19-nortestosterone 17β-acetate or as 6,6-difluoro-17α-ethynylestr-4-en-17β-ol-3-one 17β-acetate, is a steroidal progestin of the 19-nortestosterone group which was never marketed. In comparison to other steroids, is the C17β acetate ester of 6,6-difluoronorethisterone and the 6,6-difluoro analog of norethisterone acetate.

Megestrol caproate, abbreviated as MGC, is a progestin medication which was never marketed. It was developed in Russia in 2002. In animals, MGC shows 10-fold higher progestogenic activity compared to progesterone when both are administered via subcutaneous injection. In addition, MGC has no androgenic, anabolic, or estrogenic activity. The medication was suggested as a potential contraceptive and therapeutic agent.
















