Related Research Articles

Christian Friedrich Samuel Hahnemann was a German physician, best known for creating the pseudoscientific system of alternative medicine called homeopathy.

The Organon is the standard collection of Aristotle's six works on logical analysis and dialectic. The name Organon was given by Aristotle's followers, the Peripatetics.

Sophistical Refutations is a text in Aristotle's Organon in which he identified thirteen fallacies. According to Aristotle, this is the first work to treat the subject of deductive reasoning in ancient Greece.

Rapacuronium bromide is a rapidly acting, non-depolarizing aminosteroid neuromuscular blocker formerly used in modern anaesthesia, to aid and enable endotracheal intubation, which is often necessary to assist in the controlled ventilation of unconscious patients during surgery and sometimes in intensive care. As a non-depolarizing agent it did not cause initial stimulation of muscles before weakening them.

Organon of the Art of Healing by Samuel Hahnemann, 1810, laid out the doctrine of his ideas of homoeopathy. The work was repeatedly revised by Hahnemann and published in six editions, with the name changed from the second onwards to Organon of Medicine, and has been so since the mid-19th century.
Organon & Co. is an American pharmaceutical company headquartered in Jersey City, New Jersey. Organon specializes in the following core therapeutic fields: reproductive medicine, contraception, psychiatry, hormone replacement therapy (HRT), and anesthesia. Organon sells to international markets.

Farampator is an ampakine drug. It was developed by Cortex Pharmaceuticals, and licensed to Organon BioSciences for commercial development. Following the purchase of Organon by Schering-Plough in 2007, the development license to farampator was transferred. The development of farampator was eventually terminated, reportedly due to concerns about cardiac toxicity.
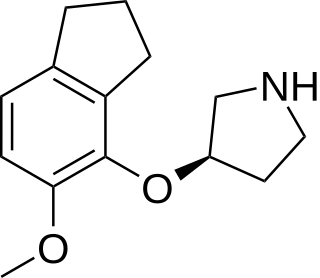
ORG-37684 is a drug developed by Organon, which acts as a potent and selective agonist for the 5-HT2 receptor family, with highest affinity at 5-HT2C and lowest at 5-HT2B subtypes. It has anorectic effects in animal studies and has been researched as a potential weight loss drug for use in humans.

The Newhouse Research Site is a drug research facility situated 15 miles (24 km) east of Glasgow in central Scotland. It is located beside the M8 motorway in Newhouse, North Lanarkshire. The site is an early drug discovery research centre with a track record of generating a succession of products in the areas of anaesthesia and psychiatry. In 2007, the Royal Society of Chemistry Malcolm Campbell Memorial Prize was awarded to researchers for its work on a new anaesthesia drug, sugammadex. It currently employs 250 scientists across a range of disciplines including medicinal chemistry, molecular biology and drug metabolism. The site is currently the largest private drug discovery centre in Scotland, and one of the biggest in the UK.
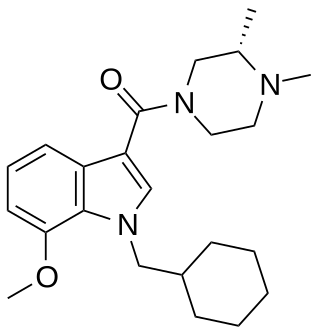
Org 28611 (SCH-900,111) is a drug developed by Organon International which acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors. It was developed with the aim of finding a water-soluble cannabinoid agonist suitable for intravenous use as an analgesic, and while it achieved this aim and has progressed as far as Phase II clinical trials in humans as both a sedative and an analgesic, results against the comparison drugs (midazolam and morphine respectively) were not particularly favourable in initial testing.

Org 28312 is a drug developed by Organon International which acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors. It was developed with the aim of finding a water-soluble cannabinoid agonist suitable for intravenous use as an analgesic, but did not proceed to human trials, with the related compound Org 28611 chosen instead due to its better penetration into the brain. The structure-activity relationships of these compounds have subsequently been investigated further leading to the development of a number of more potent analogues, derived by cyclisation around the indole or piperazine rings.
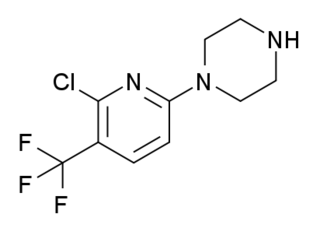
ORG-12962 is a pyridinylpiperazine drug developed by Organon, which acts as a potent and selective agonist for the 5-HT2 receptor family, with highest affinity at 5-HT2C and lowest at 5-HT2B subtypes. It was developed as a potential anti-anxiety drug, but was discontinued from human trials after tests in a public speaking challenge showed that its anti-anxiety effects were accompanied by side effects such as dizziness and a "spacey" feeling, which were attributed to poor selectivity in vivo over the hallucinogenic 5-HT2A receptor.

ORG-25435 is a synthetic drug developed by Organon International, which acts as a GABAA receptor positive allosteric modulator, and produces sedative effects. It has been researched for use as an intravenous anesthetic agent, with positive results in initial trials, although negative side effects like hypotension and tachycardia, as well as unpredictable pharmacokinetics at higher doses, have meant it has ultimately not been adopted for medical use.

ORG-26576 is an ampakine originally developed by Cortex Pharmaceuticals and then licensed to Organon International for development. In animal studies it has been shown to effectively potentiate AMPA receptor function, leading to increased BDNF release and enhanced neuronal differentiation and survival, as well as producing nootropic effects in standardised assays. Development as an antidepressant has been halted due to a failed Phase II trial for major depressive disorder.
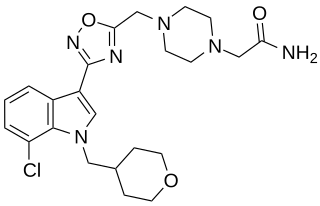
LBP-1 is a drug originally developed by Organon for the treatment of neuropathic pain, It acts as a potent and selective cannabinoid receptor agonist, with high potency at both the CB1 and CB2 receptors, but low penetration of the blood–brain barrier. This makes LBP-1 peripherally selective, and while it was effective in animal models of neuropathic pain and allodynia, it did not produce cannabinoid-appropriate responding suggestive of central effects, at any dose tested.

PTI-2 (SGT-49) is an indole-based synthetic cannabinoid. It is one of few synthetic cannabinoids containing a thiazole group and is closely related to PTI-1. These compounds may be viewed as simplified analogues of indole-3-heterocycle compounds originally developed by Organon and subsequently further researched by Merck.

PTI-1 (SGT-48) is an indole-based synthetic cannabinoid. It is one of few synthetic cannabinoids containing a thiazole group and is closely related to PTI-2. These compounds may be viewed as simplified analogues of indole-3-heterocycle compounds originally developed by Organon and subsequently further researched by Merck.

Tigestol, also known as 17α-ethynylestr-5(10)-en-17β-ol, is a steroidal progestin of the 19-nortestosterone group that was developed by Organon in the 1960s but was never marketed. It is an isomer of the related 19-nortestosterone derivative progestins lynestrenol and cingestol.
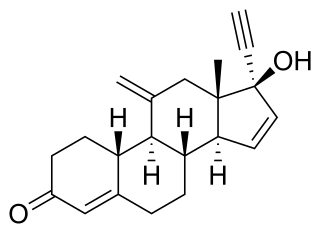
Tosagestin, also known as 11-methylene-δ15-norethisterone or 17α-ethynyl-11-methylene-19-nor-δ15-testosterone, is a progestin of the 19-nortestosterone group which was under development by Organon in the United States and Europe as a hormonal contraceptive and for the treatment of menopausal symptoms but was never marketed.
ORG-47241 is a progestin which was under development by Organon for the treatment of "female genital diseases" and as a hormonal contraceptive for the prevention of pregnancy but was never marketed.
References
- ↑ "ORG 201745 - AdisInsight". adisinsight.springer.com.