
Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.
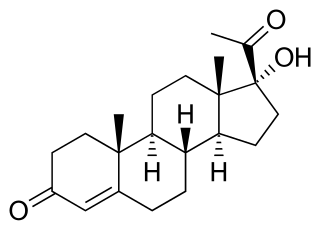
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.

Mibolerone, also known as dimethylnortestosterone (DMNT) and sold under the brand names Cheque Drops and Matenon, is a synthetic, orally active, and extremely potent anabolic–androgenic steroid (AAS) and a 17α-alkylated nandrolone (19-nortestosterone) derivative which was marketed by Upjohn for use as a veterinary drug. It was indicated specifically as an oral treatment for prevention of estrus (heat) in adult female dogs.

Hydroxyprogesterone caproate (OHPC), sold under the brand names Proluton and Makena among others, is a progestin medication which is used to prevent preterm birth in pregnant women with a history of the condition and to treat gynecological disorders. It has also been formulated in combination with estrogens for various indications and as a form of long-lasting injectable birth control. It is not used by mouth and is instead given by injection into muscle or fat, typically once per week to once per month depending on the indication.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Dimethisterone, formerly sold under the brand names Lutagan and Secrosteron among others, is a progestin medication which was used in birth control pills and in the treatment of gynecological disorders but is now no longer available. It was used both alone and in combination with an estrogen. It is taken by mouth.

Melengestrol acetate (MLGA), sold under the brand names Heifermax and MGA among others, is a progestin medication which is used in animal reproduction. It is not approved for use in humans, and is instead used as an implantable contraceptive for captive animals in zoos and other refuges, and is also used as a feed additive to promote growth in cattle, a purpose it is licensed for in the United States and Canada.

Medroxyprogesterone acetate (MPA), also known as depot medroxyprogesterone acetate (DMPA) in injectable form and sold under the brand name Depo-Provera among others, is a hormonal medication of the progestin type. It is used as a method of birth control and as a part of menopausal hormone therapy. It is also used to treat endometriosis, abnormal uterine bleeding, abnormal sexuality in males, and certain types of cancer. The medication is available both alone and in combination with an estrogen. It is taken by mouth, used under the tongue, or by injection into a muscle or fat.

Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.

Delmadinone acetate (DMA), sold under the brand name Tardak among others, is a progestin and antiandrogen which is used in veterinary medicine to treat androgen-dependent conditions such as benign prostatic hyperplasia. It must be used with care as it has the potential to cause adrenal insufficiency via inhibition of adrenocorticotropic hormone (ACTH) secretion from the pituitary gland. DMA is the C17α acetate ester of delmadinone, which, in contrast to DMA, was never marketed for medical use.

Segesterone acetate (SGA), sold under the brand names Nestorone, Elcometrine, and Annovera, is a progestin medication which is used in birth control and in the treatment of endometriosis in the United States, Brazil, and other South American countries. It is available both alone and in combination with an estrogen. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.

Hydroxyprogesterone acetate (OHPA), sold under the brand name Prodox, is an orally active progestin related to hydroxyprogesterone caproate (OHPC) which has been used in clinical and veterinary medicine. It has reportedly also been used in birth control pills.

Anagestone acetate, sold under the brand names Anatropin and Neo-Novum, is a progestin medication which was withdrawn from medical use due to carcinogenicity observed in animal studies.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

Acetomepregenol (ACM), also known as mepregenol diacetate and sold under the brand name Diamol, is a progestin medication which is used in Russia for the treatment of gynecological conditions and as a method of birth control in combination with an estrogen. It has also been studied in the treatment of threatened abortion. It has been used in veterinary medicine as well. It has been marketed since at least 1981.

17α-Methylprogesterone (17α-MP), or 17α-methylpregn-4-ene-3,20-dione, is a steroidal progestin related to progesterone that was synthesized and characterized in 1949 but was never marketed. Along with ethisterone (1938) and 19-norprogesterone (1951), 17α-MP was one of the earliest derivatives of progesterone to be identified as possessing progestogenic activity. Similarly to progesterone and derivatives like 17α-hydroxyprogesterone and 19-norprogesterone, 17α-MP was found to possess poor oral bioavailability, but showed improved progestogenic activity relative to progesterone when administered via other routes. In addition to its activity as a progestogen, 17α-MP has also been found to possess some antiglucocorticoid activity.

Flumedroxone acetate, sold under the brand names Demigran and Leomigran, is a progestin medication which is or has been used as an antimigraine agent. It is taken by mouth.

16-Methylene-17α-hydroxyprogesterone acetate is a progestin of the 17α-hydroxyprogesterone group which was never marketed. Given orally, it shows about 2.5-fold the progestogenic activity of parenteral progesterone in animal bioassays. It is a parent compound of the following clinically used progestins:
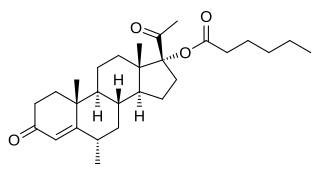
Medroxyprogesterone caproate (MPC) is a progestin and a progestogen ester which was synthesized in 1958 but was never marketed. It has been confused with hydroxyprogesterone caproate (OHPC) and medroxyprogesterone acetate (MPA) in a number of publications. In addition to MPA and OHPC, analogues of MPC include chlormadinone caproate, gestonorone caproate, megestrol caproate, and methenmadinone caproate.

6α-Methylprogesterone (6α-MP) is a progestin which was never marketed. It has 150% of the progestogenic potency of progesterone. In addition, and in contrast to progesterone, 6α-MP has weak androgenic, antiandrogenic, and synandrogenic actions. 6α-MP is structurally related to medroxyprogesterone acetate and megestrol acetate, which possess androgenic and/or antiandrogenic activity to varying degrees similarly. MPA is more androgenic than 6α-MP and MGA.




















