Progestogens, also sometimes written progestagens or gestagens, are a class of steroid hormones that bind to and activate the progesterone receptor (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.

Megestrol acetate (MGA), sold under the brand name Megace among others, is a progestin medication which is used mainly as an appetite stimulant to treat wasting syndromes such as cachexia. It is also used to treat breast cancer and endometrial cancer, and has been used in birth control. MGA is generally formulated alone, although it has been combined with estrogens in birth control formulations. It is usually taken by mouth.

Gestonorone caproate, also known as gestronol hexanoate or norhydroxyprogesterone caproate and sold under the brand names Depostat and Primostat, is a progestin medication which is used in the treatment of enlarged prostate and cancer of the endometrium. It is given by injection into muscle typically once a week.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Dimethisterone, formerly sold under the brand names Lutagan and Secrosteron among others, is a progestin medication which was used in birth control pills and in the treatment of gynecological disorders but is now no longer available. It was used both alone and in combination with an estrogen. It is taken by mouth.

Norethisterone enanthate (NETE), also known as norethindrone enanthate, is a form of progestogen-only injectable birth control which is used to prevent pregnancy in women. It may be used following childbirth, miscarriage, or abortion. The failure rate per year in preventing pregnancy is 2 per 100 women. Each dose lasts two months with only up to two doses typically recommended.

Noretynodrel, or norethynodrel, sold under the brand name Enovid among others, is a progestin medication which was previously used in birth control pills and in the treatment of gynecological disorders but is now no longer marketed. It was available both alone and in combination with an estrogen. The medication is taken by mouth.
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.

Anagestone acetate, sold under the brand names Anatropin and Neo-Novum, is a progestin medication which was withdrawn from medical use.

Ethynerone, also known as 17α-(2-chloroethynyl)estra-4,9-dien-17β-ol-3-one, is a steroidal progestin of the 19-nortestosterone group that was first reported in 1961 but was never marketed. Under the developmental code name MK-665, it was studied in combination with mestranol as an oral contraceptive. Development of the drug was discontinued due to concerns surrounding toxicity findings in dogs. It is a chloroethynylated derivative of norethisterone.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

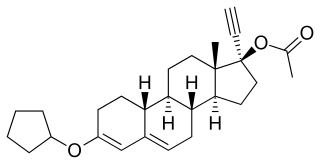
Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken by mouth.

Clomegestone acetate (USAN), or clomagestone acetate, also known as 6-chloro-17α-acetoxy-16α-methylpregna-4,6-diene-3,20-dione, is a steroidal progestin of the 17α-hydroxyprogesterone group which was developed as an oral contraceptive but was never marketed. It is the acetate ester of clomegestone, which, similarly to clomegestone acetate, was never marketed. Clomegestone acetate is also the 17-desoxy cogener of clometherone, and is somewhat more potent in comparison. Similarly to cyproterone acetate, clomegestone acetate has been found to alter insulin receptor concentrations in adipose tissue, and this may indicate the presence of glucocorticoid activity.
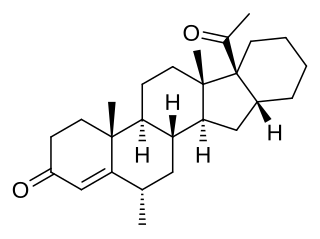
Mecigestone, also known as pentarane B, as well as 6α-methyl-16α,17α-cyclohexanoprogesterone, 6α-methylcyclohexano[1',2';16,17]pregn-4-ene-3,20-dione, or 17α-acetyl-6α-methyl-16β,24-cyclo-21-norchol-4-en-3-one, is a steroidal progestin that was developed by the Zelinskii Institute of Organic Chemistry of the Russian Academy of Sciences and has been proposed for clinical use as a progestogen but has not been marketed. It is the 6α-methylated analogue of pentarane A, which is also known as D'6-pentarane or pregna-D'6-pentarane.

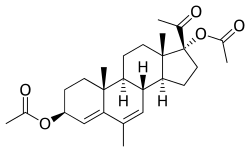
Butagest, also known as buterol, as well as 3β-hydroxy-6-methyl-17α-hydroxypregna-4,6-dien-20-one 3β-butanoate 17α-acetate or as 6-methyl-17α-hydroxy-δ6-progesterone 3β-butanoate 17α-acetate, is a steroidal progestin which was developed in Russia for potential clinical use but was never marketed. It is a modification of megestrol acetate in which the C3 ketone has been replaced with a C3β butanoyloxy moiety.

Cymegesolate, also known as cypionyl megestrol acetate or as megestrol acetate 3β-cypionate, is a progestin medication which was never marketed. It was developed in China in the late 1970s and early to mid 1980s for use as a hormonal contraceptive. The medication was formulated at a dose of 50–100 mg in combination with a "trace" dose of 0.25–0.5 mg quinestrol as a long-lasting, once-a-month combined oral contraceptive pill. This combination has been studied in 1,213 women across a total of 9,651 menstrual cycles, with contraceptive effectiveness of over 99.13% and "very few side effects." At the high dose, it showed an anovulation rate of only about 60%, and instead mediated its contraceptive effects via a marked anti-implantation effect.

Megestrol caproate, abbreviated as MGC, is a progestin medication which was never marketed. It was developed in Russia in 2002. In animals, MGC shows 10-fold higher progestogenic activity compared to progesterone when both are administered via subcutaneous injection. In addition, MGC has no androgenic, anabolic, or estrogenic activity. The medication was suggested as a potential contraceptive and therapeutic agent.

Progesterone 3-acetyl enol ether, also known as progesterone acetate, as well as 3-acetoxypregna-3,5-dien-20-one, is a progestin which was never marketed. It was reported to possess similar potency to progesterone and hydroxyprogesterone caproate in the rabbit endometrial carbonic anhydrase test, a bioassay of progestogenic activity. In addition, it was able to maintain pregnancy in animals. Progesterone 3-acetyl enol ether is closely related to quingestrone, which is also known as progesterone 3-cyclopentyl enol ether and was formerly marketed as an oral contraceptive.