| Compound | Chemical name | Structure |
|---|
| Hydroxyprogesterone | 17α-Hydroxyprogesterone |  |
| Acetomepregenol (mepregenol diacetate) | 3β,17α-Diacetoxy-6-methyl-6-dehydro-3-deketoprogesterone |  |
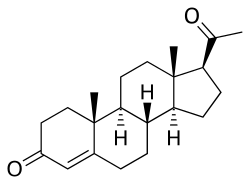
| Algestone | 16α,17α-Dihydroxyprogesterone |  |
| Algestone acetophenide | 16α,17α-Dihydroxyprogesterone acetophenide |  |
| Anagestone acetate | 17α-Acetoxy-6α-methyl-3-deketoprogesterone |  |
| Chlormadinone acetate | 17α-Acetoxy-6-chloro-6-dehydroprogesterone |  |
| Chlormethenmadinone acetate | 17α-Acetoxy-6-chloro-16-methylene-6-dehydroprogesterone |  |
| Cyproterone acetate | 17α-Acetoxy-1,2α-methylene-6-chloro-6-dehydroprogesterone |  |
| Delmadinone acetate | 17α-Acetoxy-6-chloro-1,6-didehydro-progesterone |  |
| Flugestone acetate (flurogestone acetate) | 17α-Acetoxy-9α-fluoro-11β-hydroxyprogesterone |  |
| Flumedroxone acetate | 17α-Acetoxy-6α-(trifluoromethyl)progesterone |  |
| Hydroxyprogesterone acetate | 17α-Acetoxyprogesterone |  |
| Hydroxyprogesterone caproate | 17α-Hexanoxyprogesterone |  |
| Hydroxyprogesterone heptanoate | 17α-Heptanoxyprogesterone |  |
| Medroxyprogesterone acetate | 17α-Acetoxy-6α-methylprogesterone |  |
| Megestrol acetate | 17α-Acetoxy-6-methyl-6-dehydroprogesterone |  |
| Melengestrol acetate | 17α-Acetoxy-16-methylene-6-methyl-6-dehydroprogesterone |  |
| Methenmadinone acetate | 17α-Acetoxy-16-methylene-6-dehydroprogesterone |  |
| Osaterone acetate | 17α-Acetoxy-6-chloro-2-oxa-6-dehydroprogesterone |  |
| Pentagestrone acetate | 17α-Acetoxyprogesterone 3-cyclopentyl enol ether |  |