 | |
| Clinical data | |
|---|---|
| Other names | SH-489; Metandroden; 1-Methylandrosta-1,4-diene-3,17-dione |
| Routes of administration | By mouth [1] |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
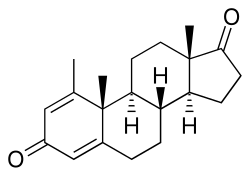
| Formula | C20H26O2 |
| Molar mass | 298.426 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Atamestane (developmental code name SH-489), also known as metandroden, as well as 1-methylandrosta-1,4-diene-3,17-dione, is a steroidal aromatase inhibitor that was studied in the treatment of cancer. [2] It blocks the production of estrogen in the body. The drug is selective, competitive, and irreversible in its inhibition of aromatase. [3] [ additional citation(s) needed ]
