
Enestebol, also known as 4-hydroxy-17α-methyl-δ1-testosterone, as well as 4,17β-dihydroxy-17α-methylandrosta-1,4-dien-3-one, is a synthetic and orally active anabolic–androgenic steroid (AAS) and a 17α-alkylated derivative of testosterone which was never marketed. It is closely related to oxymesterone (4-hydroxy-17α-methyltestosterone), as well as to chlorodehydromethyltestosterone (4-chloro-17α-methyl-δ1-testosterone), methylclostebol (4-chloro-17α-methyltestosterone), and metandienone (17α-methyl-δ1-testosterone).

Hydroxystenozole, also known as 17α-methylandrost-4-eno[3,2-c]pyrazol-17β-ol, is an orally active androgen/anabolic steroid (AAS) and a 17α-alkylated derivative of testosterone that was described in the literature in 1967 but was never marketed. It is closely related to stanozolol (17α-methyl-5α-androstano[3,2-c]pyrazol-17β-ol), differing from it only in hydrogenation.

Tiomesterone is a synthetic, orally active anabolic-androgenic steroid (AAS) and a 17α-alkylated derivative of testosterone. It was described in 1963.

Propetandrol, or propethandrol, also known as 17α-ethyl-19-nortestosterone 3β-propionate or 17α-ethyl-19-nor-4-androstenediol 3β-propionate, as well as 17α-ethylestr-4-en-3β,17β-diol 3β-propionate, is a synthetic and orally active anabolic–androgenic steroid (AAS) and progestogen and a 17α-alkylated derivative of 19-nortestosterone. It is an androgen ester – specifically, the 3β-propionate ester of norethandrolone (17α-ethyl-19-nortestosterone).
Proligestone, sold under the brand names Covinan and Delvosteron, is a progestin medication which is used in veterinary medicine.

Oxabolone is a synthetic anabolic-androgenic steroid (AAS) of the nandrolone (19-nortestosterone) group which was never marketed. It can be formulated as the cipionate ester prodrug oxabolone cipionate, which, in contrast, has been marketed for medical use.
Hydromadinone acetate, also known as chloroacetoxyprogesterone (CAP), as well as 6α-chloro-17α-acetoxyprogesterone or 6α-chloro-17α-acetoxypregn-4-ene-3,20-dione, is a steroidal progestin of the 17α-hydroxyprogesterone group, that was never marketed. It is the C17α acetate ester of hydromadinone, which, similarly, was never marketed.

Anagestone, also known as 3-deketo-6α-methyl-17α-hydroxyprogesterone or as 6α-methyl-17α-hydroxypregn-4-en-20-one, is a progestin which was never marketed.
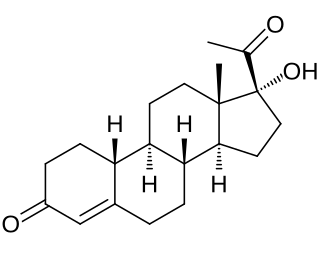
Gestronol, also known as gestonorone, as well as 17α-hydroxy-19-norprogesterone or 17α-hydroxy-19-norpregn-4-ene-3,20-dione, is a progestin of the 19-norprogesterone and 17α-hydroxyprogesterone groups which was never marketed. The C17α caproate ester of gestronol, gestonorone caproate, in contrast, has been marketed.

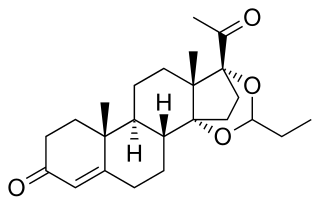
Pentagestrone, also known as 17α-hydroxyprogesterone 3-cyclopentyl enol ether, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed. An acetate ester, pentagestrone acetate, has been marketed for clinical use. Pentagestrone was described in the literature in 1960.
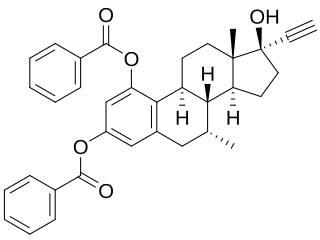
Etamestrol (INN), or eptamestrol, also known as 7α-methyl-19-nor-17α-pregna-1,3,5(10)-trien-20-yne-1,3,17-triol 1,3-dibenzoate, is a synthetic, steroidal estrogen described as an ovulation inhibitor that was synthesized in 1979 but was never marketed.
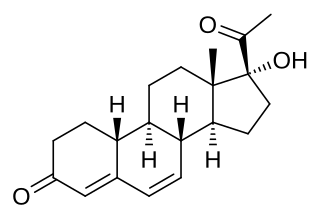
Gestadienol, also known as 6-dehydro-17α-hydroxy-19-norprogesterone, is a steroidal progestin of the 19-norprogesterone group that was never marketed.

Cismadinone (INN), also known as 6α-chloro-17α-hydroxypregna-1,4-diene-3,20-dione or 6α-chloro-δ1-dehydro-17α-hydroxyprogesterone, is a steroidal progestin closely related to the 17α-hydroxyprogesterone derivatives that was never marketed. An acetylated form, cismadinone acetate, also exists, but similarly to cismadinone, was never marketed.

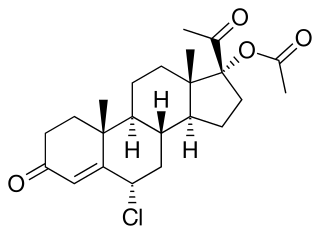
Cismadinone acetate, also known as 6α-chloro-δ1-dehydro-17α-acetoxyprogesterone or as 6α-chloro-17α-acetoxypregna-1,4-diene-3,20-dione, is a steroidal progestin related to the 17α-hydroxyprogesterone derivatives which was never marketed. It is the acetylated form of cismadinone, which is also a progestin but, similarly to cismadinone acetate, was never marketed.

Amadinone acetate, also known as 19-norchlormadinone acetate, is a steroidal progestin of the 19-norprogesterone and 17α-hydroxyprogesterone groups that was never marketed. It is the acetate ester of amadinone, which, similarly, was never marketed.

6,6-Difluoronorethisterone, also known as 6,6-difluoro-17α-ethynyl-19-nortestosterone or as 6,6-difluoro-17α-ethynylestr-4-en-17β-ol-3-one, is a steroidal progestin of the 19-nortestosterone group that was described in 1971 but was never marketed. It is a fluorinated derivative of norethisterone. The C17β acetate ester, 6,6-difluoronorethisterone acetate, has also been synthesized and described.

6,6-Difluoronorethisterone acetate, also known as 6,6-difluoro-17α-ethynyl-19-nortestosterone 17β-acetate or as 6,6-difluoro-17α-ethynylestr-4-en-17β-ol-3-one 17β-acetate, is a steroidal progestin of the 19-nortestosterone group which was never marketed. In comparison to other steroids, is the C17β acetate ester of 6,6-difluoronorethisterone and the 6,6-difluoro analog of norethisterone acetate.

Methyltestosterone 3-hexyl ether, or 17α-methyltestosterone 3-hexyl enol ether, also known as 17α-methylandrost-3,5-dien-17β-ol-3-one 3-hexyl ether, is a synthetic anabolic-androgenic steroid and an androgen ether – specifically, the 3-hexyl ether of methyltestosterone.
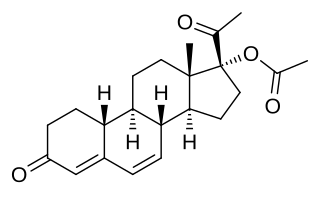
Gestadienol acetate an orally active progestin which was described in the literature in 1967 and was never marketed. It has no androgenic or estrogenic effects. The effects of gestadienol acetate on the endometrium and its general pharmacology were studied in a clinical trial in women. It has also been studied in a clinical trial for benign prostatic hyperplasia in men, but was ineffective.
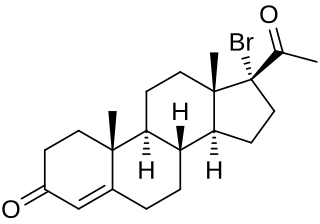
17α-Bromoprogesterone (17α-BP) is a progestin which was first described in 1957 and was never marketed. It is about twice as potent as progesterone in terms of progestogenic activity in animal bioassays. 17α-BP is a parent compound of haloprogesterone (6α-fluoro-17α-bromoprogesterone) and 6α-methyl-17α-bromoprogesterone.




















