
Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Cyproterone acetate (CPA), sold alone under the brand name Androcur or with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication used in the treatment of androgen-dependent conditions such as acne, excessive body hair growth, early puberty, and prostate cancer, as a component of feminizing hormone therapy for transgender women, and in birth control pills. It is formulated and used both alone and in combination with an estrogen. CPA is taken by mouth one to three times per day.

Cyproterone, also known by its developmental code name SH-80881, is a steroidal antiandrogen which was studied in the 1960s and 1970s but was never introduced for medical use. It is an analogue of cyproterone acetate (CPA), an antiandrogen, progestin, and antigonadotropin which was introduced instead of cyproterone and is widely used as a medication. Cyproterone and CPA were among the first antiandrogens to be developed.

Delmadinone acetate (DMA), sold under the brand name Tardak among others, is a progestin and antiandrogen which is used in veterinary medicine to treat androgen-dependent conditions such as benign prostatic hyperplasia. It must be used with care as it has the potential to cause adrenal insufficiency via inhibition of adrenocorticotropic hormone (ACTH) secretion from the pituitary gland. DMA is the C17α acetate ester of delmadinone, which, in contrast to DMA, was never marketed for medical use.

Benorterone, also known by its developmental code name SKF-7690 and as 17α-methyl-B-nortestosterone, is a steroidal antiandrogen which was studied for potential medical use but was never marketed. It was the first known antiandrogen to be studied in humans. It is taken by mouth or by application to skin.
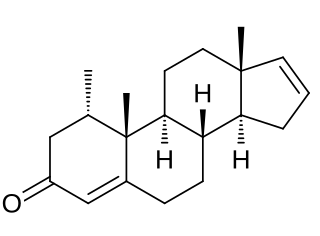
Delanterone, also known as 1α-methylandrosta-4,16-dien-3-one, is a steroidal antiandrogen described as an anti-acne agent which was never marketed. The compound showed poor efficacy as an antiandrogen in vivo in animals, suggestive of low activity or a short terminal half-life, and likely in relation to this was not further developed. It was described and characterized in the literature in 1977.

Metogest, also known as 16,16-dimethyl-19-nortestosterone, is a steroidal antiandrogen that was patented in 1975 and investigated as a treatment for acne but was never marketed.

Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine in Europe in the treatment of enlarged prostate in dogs. It is given by mouth.

Oxendolone, sold under the brand names Prostetin and Roxenone, is an antiandrogen and progestin medication which is used in Japan in the treatment of enlarged prostate. However, this use is controversial due to concerns about its clinical efficacy. Oxendolone is not effective by mouth and must be given by injection into muscle.

Topterone, also known as 17α-propyltestosterone or as 17α-propylandrost-4-en-17β-ol-3-one, is a steroidal antiandrogen that was first reported in 1978 and was developed for topical administration but, due to poor effectiveness, was never marketed.

Anagestone acetate, sold under the brand names Anatropin and Neo-Novum, is a progestin medication which was withdrawn from medical use due to carcinogenicity observed in animal studies.

Spirorenone (INN) is a steroidal antimineralocorticoid of the spirolactone group that was never marketed. Spirorenone possesses 5–8 times the antimineralocorticoid activity of spironolactone in animal studies. The initial discovery of spirorenone was deemed a great success, as no compound with greater antimineralocorticoid activity had been developed since spironolactone in 1957. Moreover, spirorenone itself has virtually no affinity for the androgen receptor while its progestogenic activity shows species differences, being somewhat greater than that of spironolactone in rabbits but absent in mice and rats. As such, it was characterized as a highly potent antimineralocorticoid with far fewer hormonal side effects relative to spironolactone.

Inocoterone acetate is a steroid-like nonsteroidal antiandrogen (NSAA) that was developed for topical administration to treat acne but was never marketed. It is the acetate ester of inocoterone, which is less potent in comparison. Inocoterone acetate is actually not a silent antagonist of the androgen receptor but rather a weak partial agonist, similarly to steroidal antiandrogens like cyproterone acetate.

Pentagestrone, also known as 17α-hydroxyprogesterone 3-cyclopentyl enol ether, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed. An acetate ester, pentagestrone acetate, has been marketed for clinical use. Pentagestrone was described in the literature in 1960.

Edogestrone, or edogesterone, also known as 17α-acetoxy-3,3-ethylenedioxy-6-methylpregn-5-en-20-one, is a steroidal progestin and antiandrogen of the 17α-hydroxyprogesterone group which was synthesized in 1964 but was never marketed. Similarly to the structurally related steroid cyproterone acetate, edogestrone binds directly to the androgen receptor and antagonizes it, displacing androgens like testosterone from the receptor, though not as potently as cyproterone acetate. The drug has also been found to suppress androgen production, likely via progesterone receptor activation-mediated antigonadotropic activity.

Amadinone (INN), also known as 19-norchlormadinone, is a steroidal progestin of the 19-norprogesterone and 17α-hydroxyprogesterone groups that was synthesized and characterized in 1968 but was never marketed. It has antigonadotropic properties, and for this reason, is a functional antiandrogen. An acetate ester, amadinone acetate, also exists, but similarly was never marketed.

BOMT, also known by its developmental code name Ro 7-2340 and as 6α-bromo-4-oxa-17α-methyl-5α-dihydrotestosterone, is a synthetic steroidal antiandrogen which was first developed in 1970 and was never marketed for medical use. It is the 6α-brominated, 4-oxygenated, and 17α-methylated derivative of the androgen dihydrotestosterone (DHT). Along with benorterone, cyproterone, and flutamide, BOMT was among the earliest antiandrogens to be developed and extensively studied, although it is less well-documented in comparison to the others. BOMT has been investigated clinically in the treatment of benign prostatic hyperplasia, though development for this use did not continue. There was also interest in BOMT for the potential applications of acne, pattern hair loss, and possibly prostate cancer, but it was not developed for these indications either.

A steroidal antiandrogen (SAA) is an antiandrogen with a steroidal chemical structure. They are typically antagonists of the androgen receptor (AR) and act both by blocking the effects of androgens like testosterone and dihydrotestosterone (DHT) and by suppressing gonadal androgen production. SAAs lower concentrations of testosterone through simulation of the negative feedback inhibition of the hypothalamus. SAAs are used in the treatment of androgen-dependent conditions in men and women, and are also used in veterinary medicine for the same purpose. They are the converse of nonsteroidal antiandrogens (NSAAs), which are antiandrogens that are not steroids and are structurally unrelated to testosterone.

AA560 is an orally active nonsteroidal antiandrogen (NSAA) that was developed in Japan and was first described in the literature in 1977 but was never marketed. It is an anilide derivative and analogue of the NSAA flutamide, and shows greater in vivo antiandrogenic potency than does flutamide. Similarly to flutamide, AA560 is a selective antagonist of the androgen receptor (AR) and consequently shows progonadotropic effects by increasing levels of gonadotropins and testosterone via disinhibition of the hypothalamic-pituitary-gonadal axis.



















