 | |
| Clinical data | |
|---|---|
| Trade names | Halotestin, Ora-Testryl, Ultandren, others |
| Other names | Fluoxymestrone; Androfluorene; NSC-12165; 9α-Fluoro-11β-hydroxy-17α-methyltestosterone; 9α-Fluoro-17α-methylandrost-4-en-11β,17β-diol-3-one |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682690 |
| Pregnancy category |
|
| Routes of administration | By mouth [1] |
| Drug class | Androgen; Anabolic steroid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 80% [3] |
| Metabolism | Liver (6β-hydroxylation, 5α- and 5β-reduction, 3α- and 3β-keto-oxidation, 11β-hydroxy-oxidation) [4] |
| Metabolites | • 5α-Dihydrofluoxymesterone [4] • 11-Oxofluoxymesterone [4] |
| Elimination half-life | 9.2 hours [5] [6] |
| Excretion | Urine (<5% unchanged) [3] [4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.875 |
| Chemical and physical data | |
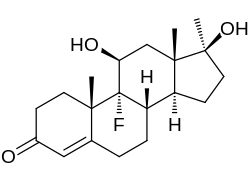
| Formula | C20H29FO3 |
| Molar mass | 336.447 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fluoxymesterone, sold under the brand names Halotestin and Ultandren among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, breast cancer in women, and anemia. [1] It is taken by mouth. [1]
Contents
- Medical uses
- Available forms
- Non-medical uses
- Side effects
- Pharmacology
- Pharmacodynamics
- Pharmacokinetics
- Chemistry
- Synthesis
- Detection in body fluids
- History
- Society and culture
- Generic names
- Brand names
- Availability
- Legal status
- References
Side effects of fluoxymesterone include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire. [1] It can also cause liver damage and cardiovascular side effects like high blood pressure. [1] [7] [8] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). [1] [9] It has strong androgenic effects and moderate anabolic effects, which make it useful for producing masculinization. [1] [10]
Fluoxymesterone was first described in 1956 and was introduced for medical use in 1957. [1] [11] In addition to its medical use, fluoxymesterone is used to improve physique and performance. [1] The drug is a controlled substance in many countries and so non-medical use is generally illicit. [1]
