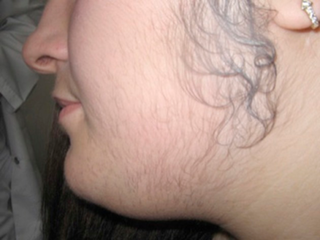
Hirsutism is excessive body hair on parts of the body where hair is normally absent or minimal. The word is from early 17th century: from Latin hirsutus meaning "hairy". It usually refers to a male pattern of hair growth in a female that may be a sign of a more serious medical condition, especially if it develops well after puberty. Cultural stigma against hirsutism can cause much psychological distress and social difficulty. Discrimination based on facial hirsutism often leads to the avoidance of social situations and to symptoms of anxiety and depression.

Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.

A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.
Feminizing hormone therapy, also known as transfeminine hormone therapy, is hormone therapy and sex reassignment therapy to change the secondary sex characteristics of transgender people from masculine or androgynous to feminine. It is a common type of transgender hormone therapy and is used to treat transgender women and non-binary transfeminine individuals. Some, in particular intersex people but also some non-transgender people, take this form of therapy according to their personal needs and preferences.

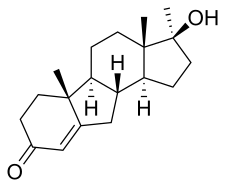
Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Cyproterone acetate (CPA), sold alone under the brand name Androcur or with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication used in the treatment of androgen-dependent conditions such as acne, excessive body hair growth, early puberty, and prostate cancer, as a component of feminizing hormone therapy for transgender women, and in birth control pills. It is formulated and used both alone and in combination with an estrogen. CPA is taken by mouth one to three times per day.

Cyproterone, also known by its developmental code name SH-80881, is a steroidal antiandrogen which was studied in the 1960s and 1970s but was never introduced for medical use. It is an analogue of cyproterone acetate (CPA), an antiandrogen, progestin, and antigonadotropin which was introduced instead of cyproterone and is widely used as a medication. Cyproterone and CPA were among the first antiandrogens to be developed.

Anagestone acetate, sold under the brand names Anatropin and Neo-Novum, is a progestin medication which was withdrawn from medical use due to carcinogenicity observed in animal studies.

Inocoterone acetate is a steroid-like nonsteroidal antiandrogen (NSAA) that was developed for topical administration to treat acne but was never marketed. It is the acetate ester of inocoterone, which is less potent in comparison. Inocoterone acetate is actually not a silent antagonist of the androgen receptor but rather a weak partial agonist, similarly to steroidal antiandrogens like cyproterone acetate.

BOMT, also known by its developmental code name Ro 7-2340 and as 6α-bromo-4-oxa-17α-methyl-5α-dihydrotestosterone, is a synthetic steroidal antiandrogen which was first developed in 1970 and was never marketed for medical use. It is the 6α-brominated, 4-oxygenated, and 17α-methylated derivative of the androgen dihydrotestosterone (DHT). Along with benorterone, cyproterone, and flutamide, BOMT was among the earliest antiandrogens to be developed and extensively studied, although it is less well-documented in comparison to the others. BOMT has been investigated clinically in the treatment of benign prostatic hyperplasia, though development for this use did not continue. There was also interest in BOMT for the potential applications of acne, pattern hair loss, and possibly prostate cancer, but it was not developed for these indications either.

Trimethyltrienolone (TMT), also known by its developmental code name R-2956 or RU-2956, is an antiandrogen medication which was never introduced for medical use but has been used in scientific research.

A steroidal antiandrogen (SAA) is an antiandrogen with a steroidal chemical structure. They are typically antagonists of the androgen receptor (AR) and act both by blocking the effects of androgens like testosterone and dihydrotestosterone (DHT) and by suppressing gonadal androgen production. SAAs lower concentrations of testosterone through simulation of the negative feedback inhibition of the hypothalamus. SAAs are used in the treatment of androgen-dependent conditions in men and women, and are also used in veterinary medicine for the same purpose. They are the converse of nonsteroidal antiandrogens (NSAAs), which are antiandrogens that are not steroids and are structurally unrelated to testosterone.

Dimethandrolone (DMA), also known by its developmental code name CDB-1321, is an experimental androgen/anabolic steroid (AAS) and progestogen medication which is under investigation for potential clinical use.

Ethyltestosterone, or 17α-ethyltestosterone, also known as 17α-ethylandrost-4-en-17β-ol-3-one or 17α-pregn-4-en-17-ol-3-one, is a synthetic, orally active anabolic–androgenic steroid (AAS) of the 17α-alkylated group related to methyltestosterone which was never marketed. Like methyltestosterone, ethyltestosterone is the parent compound of many AAS. Derivatives of ethyltestosterone include norethandrolone, ethylestrenol (ethylnandrol), norboletone, ethyldienolone, tetrahydrogestrinone, bolenol (ethylnorandrostenol), and propetandrol.
The medical uses of bicalutamide, a nonsteroidal antiandrogen (NSAA), include the treatment of androgen-dependent conditions and hormone therapy to block the effects of androgens. Indications for bicalutamide include the treatment of prostate cancer in men, skin and hair conditions such as acne, seborrhea, hirsutism, and pattern hair loss in women, high testosterone levels in women, hormone therapy in transgender women, as a puberty blocker to prevent puberty in transgender girls and to treat early puberty in boys, and the treatment of long-lasting erections in men. It may also have some value in the treatment of paraphilias and hypersexuality in men.
Comparison of the nonsteroidal antiandrogen (NSAA) bicalutamide with other antiandrogens reveals differences between the medications in terms of efficacy, tolerability, safety, and other parameters. Relative to the other first-generation NSAAs, flutamide and nilutamide, bicalutamide shows improved potency, efficacy, tolerability, and safety, and has largely replaced these medications in clinical practice. Compared to the second-generation NSAAs, enzalutamide and apalutamide, bicalutamide has inferior potency and efficacy but similar tolerability and safety and a lower propensity for drug interactions.

The pharmacology of cyproterone acetate (CPA) concerns the pharmacology of the steroidal antiandrogen and progestin medication cyproterone acetate.

Ethinylestradiol/cyproterone acetate (EE/CPA), also known as co-cyprindiol and sold under the brand names Diane and Diane-35 among others, is a combination of ethinylestradiol (EE), an estrogen, and cyproterone acetate (CPA), a progestin and antiandrogen, which is used as a birth control pill to prevent pregnancy in women. It is also used to treat androgen-dependent conditions in women such as acne, seborrhea, excessive facial/body hair growth, scalp hair loss, and high androgen levels associated with ovaries with cysts. The medication is taken by mouth once daily for 21 days, followed by a 7-day free interval.