| |
| Clinical data | |
|---|---|
| Other names | 1,4-Androstadiene-3,17-dione; 1-Dehydroandrostenedione; androsta-1,4-diene-3,17-dione; ADD |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.798 |
| Chemical and physical data | |
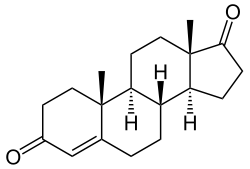
| Formula | C19H24O2 |
| Molar mass | 284.399 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Boldione, also known as androstadienedione or 1-dehydroandrostenedione, as well as 1,4-androstadiene-3,17-dione, is an important industrial precursor for various steroid hormones. [1] In the United States the chemical is regulated as a Schedule III Controlled Substance.