
Antidepressants are a class of medication used to treat major depressive disorders, anxiety disorders, chronic pain conditions and to help manage addictions. Common side-effects of antidepressants include dry mouth, weight gain, dizziness, headaches, sexual dysfunction, and emotional blunting. There is a slight increased risk of suicidal thinking and behavior when taken by children, adolescents, and young adults. Discontinuation syndrome may occur after stopping any antidepressant, which resembles recurrent depression.
An anxiolytic is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorders and their related psychological and physical symptoms.

A mood stabilizer is a psychiatric medication used to treat mood disorders characterized by intense and sustained mood shifts, such as bipolar disorder and the bipolar type of schizoaffective disorder.
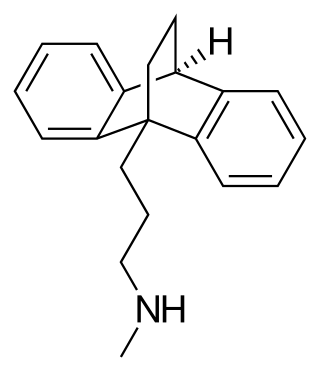
Maprotiline, sold under the brand name Ludiomil among others, is a tetracyclic antidepressant (TeCA) that is used in the treatment of depression. It may alternatively be classified as a tricyclic antidepressant (TCA), specifically a secondary amine. In terms of its chemistry and pharmacology, maprotiline is closely related to other secondary amine TCAs like nortriptyline and protriptyline, and has similar effects to them.

Duloxetine, sold under the brand name Cymbalta among others, is a medication used to treat major depressive disorder, generalized anxiety disorder, fibromyalgia, neuropathic pain and central sensitization. It is taken orally.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant drugs used to treat major depressive disorder (MDD), anxiety disorders, obsessive–compulsive disorder (OCD), social phobia, attention-deficit hyperactivity disorder (ADHD), chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the more widely used selective serotonin reuptake inhibitors (SSRIs), which act upon serotonin only.
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. The term complication is similar to adverse effect, but the latter is typically used in pharmacological contexts, or when the negative effect is expected or common. If the negative effect results from an unsuitable or incorrect dosage or procedure, this is called a medical error and not an adverse effect. Adverse effects are sometimes referred to as "iatrogenic" because they are generated by a physician/treatment. Some adverse effects occur only when starting, increasing or discontinuing a treatment. Adverse effects can also be caused by placebo treatments . Using a drug or other medical intervention which is contraindicated may increase the risk of adverse effects. Adverse effects may cause complications of a disease or procedure and negatively affect its prognosis. They may also lead to non-compliance with a treatment regimen. Adverse effects of medical treatment resulted in 142,000 deaths in 2013 up from 94,000 deaths in 1990 globally.

Nefazodone, sold formerly under the brand names Serzone, Dutonin, and Nefadar among others, is an atypical antidepressant which was first marketed by Bristol-Myers Squibb (BMS) in 1994. BMS withdrew the brand name Serzone from the market by 2004 due to decreasing sales due to the rare incidence of severe liver damage and the onset of generic competition. Generic versions were introduced in 2003. The incidence of severe liver damage is approximately 1 in every 250,000 to 300,000 patient-years.
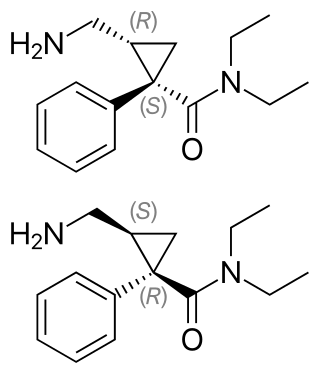
Milnacipran is a serotonin–norepinephrine reuptake inhibitor (SNRI) used in the clinical treatment of fibromyalgia. It is not approved for the clinical treatment of major depressive disorder in the US, but it is in other countries.

Moclobemide, sold under the brand names Amira, Aurorix, Clobemix, Depnil and Manerix among others, is a reversible inhibitor of monoamine oxidase A (RIMA) drug primarily used to treat depression and social anxiety. It is not approved for use in the United States, but is approved in other Western countries such as Canada, the UK and Australia. It is produced by affiliates of the Hoffmann–La Roche pharmaceutical company. Initially, Aurorix was also marketed by Roche in South Africa, but was withdrawn after its patent rights expired and Cipla Medpro's Depnil and Pharma Dynamic's Clorix became available at half the cost.
Toxic encephalopathy is a neurologic disorder caused by exposure to neurotoxic organic solvents such as toluene, following exposure to heavy metals such as manganese, as a side effect of melarsoprol treatment for African trypanosomiasis, adverse effects to prescription drugs, or exposure to extreme concentrations of any natural toxin such as cyanotoxins found in shellfish or freshwater cyanobacteria crusts. Toxic encephalopathy can occur following acute or chronic exposure to neurotoxicants, which includes all natural toxins. Exposure to toxic substances can lead to a variety of symptoms, characterized by an altered mental status, memory loss, and visual problems. Toxic encephalopathy can be caused by various chemicals, some of which are commonly used in everyday life, or cyanotoxins which are bio-accumulated from harmful algal blooms (HABs) which have settled on the benthic layer of a waterbody. Toxic encephalopathy can permanently damage the brain and currently treatment is mainly just for the symptoms.

Agomelatine, sold under the brand names Valdoxan and Thymanax, among others, is an atypical antidepressant most commonly used to treat major depressive disorder and generalized anxiety disorder. One review found that it is as effective as other antidepressants with similar discontinuation rates overall but less discontinuations due to side effects. Another review also found it was similarly effective to many other antidepressants.
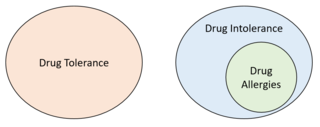
Drug intolerance or drug sensitivity refers to an inability to tolerate the adverse effects of a medication, generally at therapeutic or subtherapeutic doses. Conversely, a patient is said to be "tolerating" a drug when they can tolerate its adverse effects. Some instances of drug intolerance are known to result from genetic variations in drug metabolism.

Antimigraine drugs are medications intended to reduce the effects or intensity of migraine headache. They include drugs for the treatment of acute migraine symptoms as well as drugs for the prevention of migraine attacks.

Vilazodone, sold under the brand name Viibryd among others, is a medication used to treat major depressive disorder. It is taken by mouth.
A glossary of terms used in clinical research.
Naluzotan is a serotonergic drug of the phenylpiperazine class that was under investigation by EPIX Pharmaceuticals Inc for the treatment of generalized anxiety disorder and major depressive disorder. It acts as a selective and potent 5-HT1A receptor partial agonist, readily stimulating prolactin responses, though it has also been found to bind to and activate the σ receptor. Naluzotan was well tolerated in clinical trials, with more patients in the control group dropping out due to adverse effects than in the active group in one study. The most frequently reported side effect was headache in 15% of patients. In addition, naluzotan demonstrated significant antidepressant and anxiolytic effects as per the HAM-D and MADRS and the HAM-A, respectively, in some trials, but in others it did not. In the end it was not found to be significantly superior enough to placebo and development was stopped.

Amitriptylinoxide, or amitriptyline N-oxide, is a tricyclic antidepressant (TCA) which was introduced in Europe in the 1970s for the treatment of depression.
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.
Selective serotonin reuptake inhibitors, or serotonin-specific re-uptake inhibitor (SSRIs), are a class of chemical compounds that have contributed to the major advances as antidepressants where they have revolutionised the treatment of depression and other psychiatric disorders. The SSRIs are therapeutically useful in the treatment of panic disorder (PD), posttraumatic stress disorder (PTSD), social anxiety disorder, obsessive-compulsive disorder (OCD), premenstrual dysphoric disorder (PMDD), and anorexia. There is also clinical evidence of SSRIs efficiency in the treatment of the negative symptoms of schizophrenia and their ability to prevent cardiovascular diseases.












