Related Research Articles

An analgesic or painkiller is any member of the group of drugs used to achieve analgesia, relief from pain.

Pharmacology is a branch of pharmaceutical sciences which is concerned with the study of drug or medication action, where a drug can be broadly or narrowly defined as any man-made, natural, or endogenous molecule which exerts a biochemical or physiological effect on the cell, tissue, organ, or organism. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.
The therapeutic index is a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes toxicity.The related terms therapeutic window or safety window refer to a range of doses which optimize between efficacy and toxicity, achieving the greatest therapeutic benefit without resulting in unacceptable side-effects or toxicity.
Neurotoxicity is a form of toxicity in which a biological, chemical, or physical agent produces an adverse effect on the structure or function of the central and/or peripheral nervous system. It occurs when exposure to a substance – specifically, a neurotoxin or neurotoxicant– alters the normal activity of the nervous system in such a way as to cause permanent or reversible damage to nervous tissue. This can eventually disrupt or even kill neurons, which are cells that transmit and process signals in the brain and other parts of the nervous system. Neurotoxicity can result from organ transplants, radiation treatment, certain drug therapies, recreational drug use, and exposure to heavy metals, bites from certain species of venomous snakes, pesticides, certain industrial cleaning solvents, fuels and certain naturally occurring substances. Symptoms may appear immediately after exposure or be delayed. They may include limb weakness or numbness, loss of memory, vision, and/or intellect, uncontrollable obsessive and/or compulsive behaviors, delusions, headache, cognitive and behavioral problems and sexual dysfunction. Chronic mold exposure in homes can lead to neurotoxicity which may not appear for months to years of exposure. All symptoms listed above are consistent with mold mycotoxin accumulation.

Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs. The effects can include those manifested within animals, microorganisms, or combinations of organisms.
A prodrug is a medication or compound that, after administration, is metabolized into a pharmacologically active drug. Inactive prodrugs are pharmacologically inactive medications that are metabolized into an active form within the body. Instead of administering a drug directly, a corresponding prodrug might be used instead to improve how a medicine is absorbed, distributed, metabolized, and excreted (ADME).
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. If it results from an unsuitable or incorrect dosage or procedure, this is called a medical error and not a complication. Adverse effects are sometimes referred to as "iatrogenic" because they are generated by a physician/treatment. Some adverse effects occur only when starting, increasing or discontinuing a treatment.
A biological target is anything within a living organism to which some other entity is directed and/or binds, resulting in a change in its behavior or function. Examples of common classes of biological targets are proteins and nucleic acids. The definition is context-dependent, and can refer to the biological target of a pharmacologically active drug compound, the receptor target of a hormone, or some other target of an external stimulus. Biological targets are most commonly proteins such as enzymes, ion channels, and receptors.

Anthracyclines is a class of drugs used in cancer chemotherapy that are extracted from Streptomyces bacterium. These compounds are used to treat many cancers, including leukemias, lymphomas, breast, stomach, uterine, ovarian, bladder cancer, and lung cancers. The first anthracycline discovered was daunorubicin, which is produced naturally by Streptomyces peucetius, a species of actinobacteria. Clinically the most important anthracyclines are doxorubicin, daunorubicin, epirubicin and idarubicin.

Erlotinib, sold under the brand name Tarceva among others, is a medication used to treat non-small cell lung cancer (NSCLC) and pancreatic cancer. Specifically it is used for NSCLC with mutations in the epidermal growth factor receptor (EGFR) — either an exon 19 deletion (del19) or exon 21 (L858R) substitution mutation — which has spread to other parts of the body. It is taken by mouth.
A toxicophore is a chemical structure or a portion of a structure that is related to the toxic properties of a chemical. Toxicophores can act directly or can require metabolic activation.

In pharmacology, the term mechanism of action (MOA) refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect. A mechanism of action usually includes mention of the specific molecular targets to which the drug binds, such as an enzyme or receptor. Receptor sites have specific affinities for drugs based on the chemical structure of the drug, as well as the specific action that occurs there.
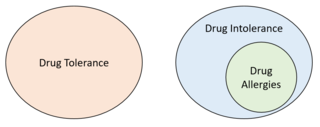
Drug intolerance or drug sensitivity refers to an inability to tolerate the adverse effects of a medication, generally at therapeutic or subtherapeutic doses. Conversely, a patient is said to be "tolerating" a drug when they can tolerate its adverse effects. It is not to be confused with a drug allergy, which is a form of drug intolerance, but requires an immune-mediated component. It is also not to be confused with drug tolerance which refers to a lack of adverse effects even at higher than average doses. Some instances of drug intolerance are known to result from genetic variations in drug metabolism.

Pazopanib is a potent and selective multi-targeted receptor tyrosine kinase inhibitor that blocks tumour growth and inhibits angiogenesis. It has been approved for renal cell carcinoma and soft tissue sarcoma by numerous regulatory administrations worldwide.
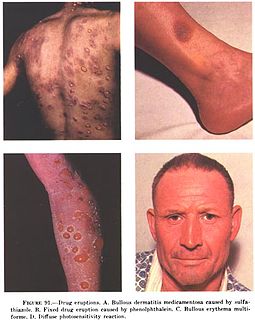
In medicine, a drug eruption is an adverse drug reaction of the skin. Most drug-induced cutaneous reactions are mild and disappear when the offending drug is withdrawn. These are called "simple" drug eruptions. However, more serious drug eruptions may be associated with organ injury such as liver or kidney damage and are categorized as "complex". Drugs can also cause hair and nail changes, affect the mucous membranes, or cause itching without outward skin changes.

Reproductive toxicity is a hazard associated with some chemical substances, which interfere in some way with normal reproduction; such substances are called reprotoxic. They may adversely affect sexual function and fertility in adult males and females, as well as causing developmental toxicity in the offspring. Reproductive toxicity is usually defined practically, to include several different effects which are unrelated to each other except in their outcome of lowered effective fertility. The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) separates reproductive toxicity from germ cell mutagenicity and carcinogenicity, even though both these hazards may also affect fertility.
Arsenic biochemistry refers to biochemical processes that can use arsenic or its compounds, such as arsenate. Arsenic is a moderately abundant element in Earth's crust, and although many arsenic compounds are often considered highly toxic to most life, a wide variety of organoarsenic compounds are produced biologically and various organic and inorganic arsenic compounds are metabolized by numerous organisms. This pattern is general for other related elements, including selenium, which can exhibit both beneficial and deleterious effects. Arsenic biochemistry has become topical since many toxic arsenic compounds are found in some aquifers, potentially affecting many millions of people via biochemical processes.
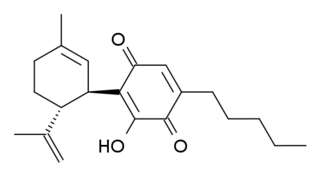
HU-331 is a quinone anticarcinogenic drug synthesized from cannabidiol, a cannabinoid in the Cannabis sativa plant. It showed a great efficacy against oncogenic human cells. HU-331 does not cause arrest in cell cycle, cell apoptosis or caspase activation. HU-331 inhibits DNA topoisomerase II even at nanomolar concentrations, but has shown a negligible effect on the action of DNA topoisomerase I. The cannabinoid quinone HU-331 is a very specific inhibitor of topoisomerase II, compared with most known anticancer quinones. One of the main objectives of these studies is the development of a new quinone derived compound that produces anti-neoplastic activity while maintaining low toxicity at therapeutic doses.
Toxicodynamics, termed pharmacodynamics in pharmacology, describes the dynamic interactions of a toxicant with a biological target and its biological effects. A biological target, also known as the site of action, can be binding proteins, ion channels, DNA, or a variety of other receptors. When a toxicant enters an organism, it can interact with these receptors and produce structural or functional alterations. The mechanism of action of the toxicant, as determined by a toxicant’s chemical properties, will determine what receptors are targeted and the overall toxic effect at the cellular level and organismal level.
This article is about gold nanoparticles in chemotherapy and radiotherapy. For colloidal gold, see colloidal gold.
References
- 1 2 Guengerich, F. Peter (2011). "Mechanisms of Drug Toxicity and Relevance to Pharmaceutical Development". Drug Metabolism and Pharmacokinetics. 26 (1): 3–14. doi:10.2133/dmpk.DMPK-10-RV-062. PMC 4707670 . PMID 20978361.
- ↑ Rodenas, M.C.; Cabas, I.; Abellán, E.; Meseguer, J.; Mulero, V.; García-Ayala, A. (December 2015). "Tamoxifen persistently disrupts the humoral adaptive immune response of gilthead seabream (Sparus aurata L.)". Developmental & Comparative Immunology. 53 (2): 283–292. doi:10.1016/j.dci.2015.06.014. PMID 26234710.
- ↑ Rudmann, Daniel G. (2013). "On-target and off-target-based toxicologic effects". Toxicologic Pathology. 41 (2): 310–314. doi: 10.1177/0192623312464311 . PMID 23085982.
- ↑ Hussar, Daniel A. "Drug Interactions". Merck Manuals Consumer Version.
- 1 2 Alam, Khondoker; Pahwa, Sonia; Wang, Xueying; Zhang, Pengyue; Ding, Kai; Abuznait, Alaa H.; Li, Lang; Yue, Wei (2016). "Downregulation of organic anion transporting polypeptide (OATP) 1B1 transport function by lysosomotropic drug chloroquine: implication of OATP-mediated drug-drug interactions". Molecular Pharmaceutics. 13 (3): 839–851. doi:10.1021/acs.molpharmaceut.5b00763. PMC 4970216 . PMID 26750564.
- ↑ Klaassen, Curtis D., ed. (2013). Casarett and Doull's toxicology : the basic science of poisons (8th ed.). New York: McGraw-Hill Education. ISBN 978-0-07-176923-5.
- 1 2 3 4 Boelsterli, Urs A. (2002). Mechanistic Toxicology the Molecular Basis of How Chemicals Disrupt Biological. London: Taylor & Francis. ISBN 0-203-36176-8.
- 1 2 Long, Scott. "Toxicity of Selected Drugs". faculty.swosu.edu.
- ↑ Rosa, Gian Marco; Gigli, Lorenzo; Tagliasacchi, Maria Isabella; Di Iorio, Cecilia; Carbone, Federico; Nencioni, Alessio; Montecucco, Fabrizio; Brunelli, Claudio (March 2016). "Update on cardiotoxicity of anti-cancer treatments". European Journal of Clinical Investigation. 46 (3): 264–284. doi: 10.1111/eci.12589 . PMID 26728634.