 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Elidel |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | topical |
| Drug class | immunosuppressant |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | low systemic absorption |
| Protein binding | 74%–87% |
| Metabolism | Hepatic CYP3A |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.124.895 |
| Chemical and physical data | |
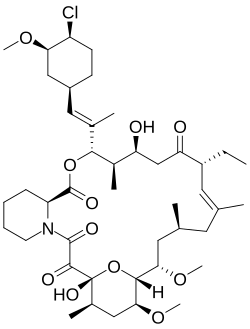
| Formula | C43H68ClNO11 |
| Molar mass | 810.46 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pimecrolimus is an immunosuppressant drug of the calcineurin inhibitor class used in the treatment of atopic dermatitis (eczema).
Contents
- Medical uses
- Atopic dermatitis
- Side effects
- Pharmacology
- Development and production
- References
- External links
It is available as a topical cream. It was developed and formerly marketed by Novartis under the trade name Elidel.