| CYP17A1 inhibitor | |
|---|---|
| Drug class | |
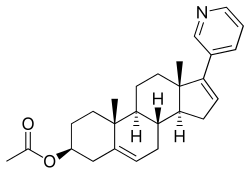 Abiraterone acetate, a steroidal CYP17A1 inhibitor that is used in the treatment of prostate cancer. | |
| Class identifiers | |
| Synonyms | Androgen synthesis inhibitors |
| Use | Prostate cancer, precocious puberty, breast cancer, others |
| ATC code | L02BX |
| Biological target | CYP17A1 |
| Chemical class | Steroidal; Nonsteroidal |
| Legal status | |
| In Wikidata | |
A CYP17A1 inhibitor is a type of drug that inhibits the enzyme CYP17A1. [1] CYP17A1 inhibitors work by blocking specific enzyme functions, impacting androgen biosynthesis.