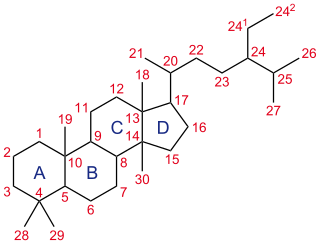
A steroid is an organic compound with four fused rings arranged in a specific molecular configuration.

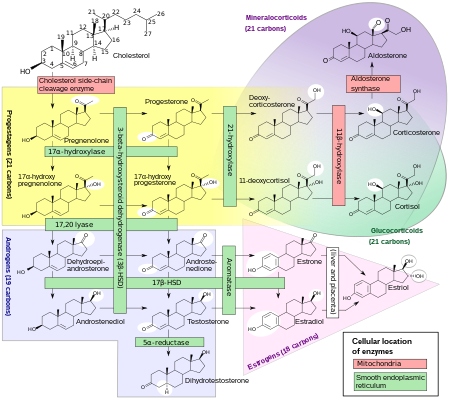
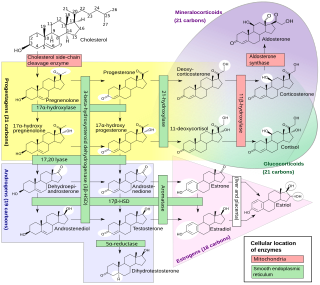
Progestogens, also sometimes written progestins, progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.

The adrenal cortex is the outer region and also the largest part of the adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is also a secondary site of androgen synthesis.

Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders characterized by impaired cortisol synthesis. It results from the deficiency of one of the five enzymes required for the synthesis of cortisol in the adrenal cortex. Most of these disorders involve excessive or deficient production of hormones such as glucocorticoids, mineralocorticoids, or sex steroids, and can alter development of primary or secondary sex characteristics in some affected infants, children, or adults. It is one of the most common autosomal recessive disorders in humans.

Lipoid congenital adrenal hyperplasia is an endocrine disorder that is an uncommon and potentially lethal form of congenital adrenal hyperplasia (CAH). It arises from defects in the earliest stages of steroid hormone synthesis: the transport of cholesterol into the mitochondria and the conversion of cholesterol to pregnenolone—the first step in the synthesis of all steroid hormones. Lipoid CAH causes mineralocorticoid deficiency in affected infants and children. Male infants are severely undervirilized causing their external genitalia to look feminine. The adrenals are large and filled with lipid globules derived from cholesterol.
Congenital adrenal hyperplasia due to 17α-hydroxylase deficiency is an uncommon form of congenital adrenal hyperplasia (CAH) resulting from a mutation in the gene CYP17A1, which produces the enzyme 17α-hydroxylase. It causes decreased synthesis of cortisol and sex hormones, with resulting increase in mineralocorticoid production. Thus, common symptoms include mild cortisol deficiency, ambiguous genitalia in men or amenorrhea at puberty in women, and hypokalemic hypertension. However, partial (incomplete) deficiency often has inconsistent symptoms between patients, and affected women may be asymptomatic except for infertility.
11β-Hydroxysteroid dehydrogenase enzymes catalyze the conversion of inert 11 keto-products (cortisone) to active cortisol, or vice versa, thus regulating the access of glucocorticoids to the steroid receptors.

Cytochrome P450 17A1 is an enzyme of the hydroxylase type that in humans is encoded by the CYP17A1 gene on chromosome 10. It is ubiquitously expressed in many tissues and cell types, including the zona reticularis and zona fasciculata of the adrenal cortex as well as gonadal tissues. It has both 17α-hydroxylase and 17,20-lyase activities, and is a key enzyme in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens. More specifically, the enzyme acts upon pregnenolone and progesterone to add a hydroxyl (-OH) group at carbon 17 position (C17) of the steroid D ring, or acts upon 17α-hydroxyprogesterone and 17α-hydroxypregnenolone to split the side-chain off the steroid nucleus.
3β-Hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD) is an enzyme that catalyzes the biosynthesis of the steroid progesterone from pregnenolone, 17α-hydroxyprogesterone from 17α-hydroxypregnenolone, and androstenedione from dehydroepiandrosterone (DHEA) in the adrenal gland. It is the only enzyme in the adrenal pathway of corticosteroid synthesis that is not a member of the cytochrome P450 family. It is also present in other steroid-producing tissues, including the ovary, testis and placenta. In humans, there are two 3β-HSD isozymes encoded by the HSD3B1 and HSD3B2 genes.
An inborn error of steroid metabolism is an inborn error of metabolism due to defects in steroid metabolism.
A neurosteroidogenesis inhibitor is a drug that inhibits the production of endogenous neurosteroids. Neurosteroids include the excitatory neurosteroids pregnenolone sulfate, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEA-S), and the inhibitory neurosteroids allopregnanolone, tetrahydrodeoxycorticosterone (THDOC), and 3α-androstanediol, among others. By inhibiting the synthesis of endogenous neurosteroids, neurosteroidogenesis inhibitors have effects in the central nervous system.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.
An androgen synthesis inhibitor is a type of drug which inhibits the enzymatic synthesis of androgens, such as testosterone and dihydrotestosterone (DHT). They include:
Adrenal steroids are steroids that are derived from the adrenal glands. They include corticosteroids, which consist of glucocorticoids like cortisol and mineralocorticoids like aldosterone, adrenal androgens like dehydroepiandrosterone (DHEA), DHEA sulfate (DHEA-S), and androstenedione (A4), and neurosteroids like DHEA and DHEA-S, as well as pregnenolone and pregnenolone sulfate (P5-S). Adrenal steroids are specifically produced in the adrenal cortex.

Amphenone B, or simply amphenone, also known as 3,3-bis(p-aminophenyl)butan-2-one, is an inhibitor of steroid hormone and thyroid hormone biosynthesis which was never marketed but has been used as a tool in scientific research to study corticosteroids and the adrenal glands. It acts as competitive inhibitor of 11β-hydroxylase, 17α-hydroxylase, 17,20-lyase, 21-hydroxylase, and 3β-hydroxysteroid dehydrogenase, as well as of cholesterol side-chain cleavage enzyme, thereby inhibiting the production of steroid hormones including glucocorticoids, mineralocorticoids, androgens, and estrogens. In addition, amphenone B inhibits the production of thyroxine by a thiouracil-like mechanism, specifically via inhibition of organic binding of iodine and uptake of iodide by the thyroid gland.

5α-Dihydronorethisterone is a major active metabolite of norethisterone (norethindrone). Norethisterone is a progestin with additional weak androgenic and estrogenic activity. 5α-DHNET is formed from norethisterone by 5α-reductase in the liver and other tissues.
An estrogen synthesis inhibitor is a type of drug which inhibits the enzymatic synthesis of estrogens, such as estradiol. They include:

The androgen backdoor pathway is responsible for the synthesis of physiologically relevant androgens. This process starts with 21-carbon steroids, also known as pregnanes, and involves a step called "5α-reduction". Notably, this pathway does not require the intermediate formation of testosterone, hence the term "bypassing testosterone" is sometimes used in medical literature as the hallmark feature of this way of androgen biosynthesis. This feature is a key distinction from the conventional, canonical androgenic pathway, which necessitates the involvement of testosterone as an intermediate in the synthesis of androgens.
A progesterone synthesis inhibitor, or progestogen synthesis inhibitor, is a type of drug which inhibits the enzymatic synthesis of progesterone. They include: