
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues. However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors, and acting as a neurosteroid and modulator of neurotrophic factor receptors.

Estriol (E3), also spelled oestriol, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estrone. Levels of estriol in women who are not pregnant are almost undetectable. However, during pregnancy, estriol is synthesized in very high quantities by the placenta and is the most produced estrogen in the body by far, although circulating levels of estriol are similar to those of other estrogens due to a relatively high rate of metabolism and excretion. Relative to estradiol, both estriol and estrone have far weaker activity as estrogens.
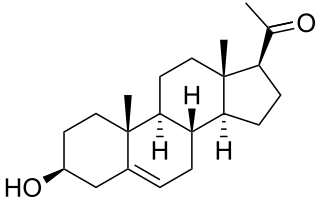
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids. In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.

Dehydroepiandrosterone sulfate, abbreviated as DHEA sulfate or DHEA-S, also known as androstenolone sulfate, is an endogenous androstane steroid that is produced by the adrenal cortex. It is the 3β-sulfate ester and a metabolite of dehydroepiandrosterone (DHEA) and circulates in far greater relative concentrations than DHEA. The steroid is hormonally inert and is instead an important neurosteroid and neurotrophin.

Steroid sulfatase (STS), or steryl-sulfatase, formerly known as arylsulfatase C, is a sulfatase enzyme involved in the metabolism of steroids. It is encoded by the STS gene.

Alcohol sulfotransferase is an enzyme that catalyzes the sulfate conjugation of primary and secondary alcohols including many hormones, neurotransmitters, drugs, and xenobiotic compounds.
An aryl sulfotransferase is an enzyme that transfers a sulfate group from phenolic sulfate esters to a phenolic acceptor substrate.
In enzymology, a cortisol sulfotransferase is an enzyme that catalyzes the chemical reaction
Estrone sulfotransferase (EST), also known as estrogen sulfotransferase, is an enzyme that catalyzes the transformation of an unconjugated estrogen like estrone into a sulfated estrogen like estrone sulfate. It is a steroid sulfotransferase and belongs to the family of transferases, to be specific, the sulfotransferases, which transfer sulfur-containing groups. This enzyme participates in androgen and estrogen metabolism and sulfur metabolism.
In enzymology, a [heparan sulfate]-glucosamine 3-sulfotransferase 3 is an enzyme that catalyzes the chemical reaction

Estrogen sulfotransferase is an enzyme that in humans is encoded by the SULT1E1 gene.

Sulfotransferase family cytosolic 2B member 1 is an enzyme that in humans is encoded by the SULT2B1 gene.

Estrone sulfate, also known as E1S, E1SO4 and estrone 3-sulfate, is a natural, endogenous steroid and an estrogen ester and conjugate.

Bile salt sulfotransferase also known as hydroxysteroid sulfotransferase (HST) or sulfotransferase 2A1 (ST2A1) is an enzyme that in humans is encoded by the SULT2A1 gene.
Sulfate conjugates are a heterogeneous class of polar, anionic organosulfate compounds containing an ester of sulfuric acid. Sulfate conjugates commonly result from the metabolic conjugation of endogenous and exogenous compounds with sulfate (-OSO3−).

Quercetin 3-sulfate is a plasma human metabolite of quercetin. It is the sulfate conjugate of quercetin.
N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase is an enzyme with systematic name 3'-phosphoadenylyl-sulfate:(dermatan)-4-O-sulfo-N-acetyl-D-galactosamine 6-O-sulfotransferase. This enzyme catalyses the following chemical reaction
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.

Estradiol sulfate (E2S), or 17β-estradiol 3-sulfate, is a natural, endogenous steroid and an estrogen ester. E2S itself is biologically inactive, but it can be converted by steroid sulfatase into estradiol, which is a potent estrogen. Simultaneously, estrogen sulfotransferases convert estradiol to E2S, resulting in an equilibrium between the two steroids in various tissues. Estrone and E2S are the two immediate metabolic sources of estradiol. E2S can also be metabolized into estrone sulfate (E1S), which in turn can be converted into estrone and estradiol. Circulating concentrations of E2S are much lower than those of E1S. High concentrations of E2S are present in breast tissue, and E2S has been implicated in the biology of breast cancer via serving as an active reservoir of estradiol.

Steroid sulfates are endogenous sulfate esters of steroids. They are formed by steroid sulfotransferases via sulfation of endogenous steroids like cholesterol and steroid hormones. Although steroid sulfates do not bind to steroid hormone receptors and hence are hormonally inert, they can be desulfated by steroid sulfatase and in this way serve as precursors and circulating reservoirs for their active unsulfated counterparts. In addition, some steroid sulfates have biological activity in their own right, for instance acting as neurosteroids and modulating ligand-gated ion channels such as the GABAA and NMDA receptors among other biological targets.












