Glycolysis is the metabolic pathway that converts glucose into pyruvate and, in most organisms, occurs in the liquid part of cells. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

In biochemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology. Protein phosphorylation often activates many enzymes.
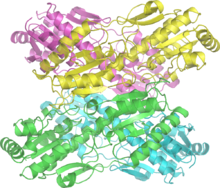
A hexokinase is an enzyme that irreversibly phosphorylates hexoses, forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexokinase possesses the ability to transfer an inorganic phosphate group from ATP to a substrate.

Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises the concentration of glucose and fatty acids in the bloodstream and is considered to be the main catabolic hormone of the body. It is also used as a medication to treat a number of health conditions. Its effect is opposite to that of insulin, which lowers extracellular glucose. It is produced from proglucagon, encoded by the GCG gene.
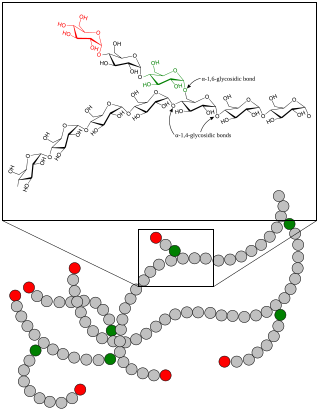
Glycogenolysis is the breakdown of glycogen (n) to glucose-1-phosphate and glycogen (n-1). Glycogen branches are catabolized by the sequential removal of glucose monomers via phosphorolysis, by the enzyme glycogen phosphorylase.
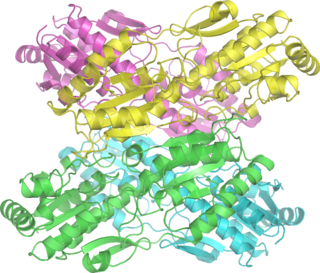
Phosphofructokinase-1 (PFK-1) is one of the most important regulatory enzymes of glycolysis. It is an allosteric enzyme made of 4 subunits and controlled by many activators and inhibitors. PFK-1 catalyzes the important "committed" step of glycolysis, the conversion of fructose 6-phosphate and ATP to fructose 1,6-bisphosphate and ADP. Glycolysis is the foundation for respiration, both anaerobic and aerobic. Because phosphofructokinase (PFK) catalyzes the ATP-dependent phosphorylation to convert fructose-6-phosphate into fructose 1,6-bisphosphate and ADP, it is one of the key regulatory steps of glycolysis. PFK is able to regulate glycolysis through allosteric inhibition, and in this way, the cell can increase or decrease the rate of glycolysis in response to the cell's energy requirements. For example, a high ratio of ATP to ADP will inhibit PFK and glycolysis. The key difference between the regulation of PFK in eukaryotes and prokaryotes is that in eukaryotes PFK is activated by fructose 2,6-bisphosphate. The purpose of fructose 2,6-bisphosphate is to supersede ATP inhibition, thus allowing eukaryotes to have greater sensitivity to regulation by hormones like glucagon and insulin.

5' AMP-activated protein kinase or AMPK or 5' adenosine monophosphate-activated protein kinase is an enzyme that plays a role in cellular energy homeostasis, largely to activate glucose and fatty acid uptake and oxidation when cellular energy is low. It belongs to a highly conserved eukaryotic protein family and its orthologues are SNF1 in yeast, and SnRK1 in plants. It consists of three proteins (subunits) that together make a functional enzyme, conserved from yeast to humans. It is expressed in a number of tissues, including the liver, brain, and skeletal muscle. In response to binding AMP and ADP, the net effect of AMPK activation is stimulation of hepatic fatty acid oxidation, ketogenesis, stimulation of skeletal muscle fatty acid oxidation and glucose uptake, inhibition of cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibition of adipocyte lipogenesis, inhibition of adipocyte lipolysis, and modulation of insulin secretion by pancreatic β-cells.

Glucose 6-phosphate is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this way.
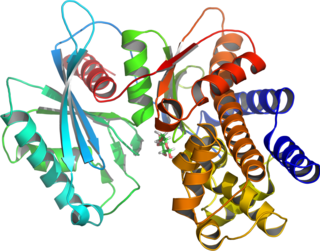
Glucokinase is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate. Glucokinase occurs in cells in the liver and pancreas of humans and most other vertebrates. In each of these organs it plays an important role in the regulation of carbohydrate metabolism by acting as a glucose sensor, triggering shifts in metabolism or cell function in response to rising or falling levels of glucose, such as occur after a meal or when fasting. Mutations of the gene for this enzyme can cause unusual forms of diabetes or hypoglycemia.
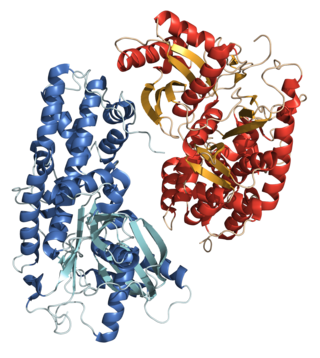
The glucokinase regulatory protein (GKRP) also known as glucokinase regulator (GCKR) is a protein produced in hepatocytes. GKRP binds and moves glucokinase (GK), thereby controlling both activity and intracellular location of this key enzyme of glucose metabolism.
Glycogenesis is the process of glycogen synthesis, in which glucose molecules are added to chains of glycogen for storage. This process is activated during rest periods following the Cori cycle, in the liver, and also activated by insulin in response to high glucose levels.

Glycogen phosphorylase is one of the phosphorylase enzymes. Glycogen phosphorylase catalyzes the rate-limiting step in glycogenolysis in animals by releasing glucose-1-phosphate from the terminal alpha-1,4-glycosidic bond. Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation and allosteric effects.
Glucose transporter type 4 (GLUT4), also known as solute carrier family 2, facilitated glucose transporter member 4, is a protein encoded, in humans, by the SLC2A4 gene. GLUT4 is the insulin-regulated glucose transporter found primarily in adipose tissues and striated muscle. The first evidence for this distinct glucose transport protein was provided by David James in 1988. The gene that encodes GLUT4 was cloned and mapped in 1989.
The glucose cycle occurs primarily in the liver and is the dynamic balance between glucose and glucose 6-phosphate. This is important for maintaining a constant concentration of glucose in the blood stream.

Fructose 2,6-bisphosphate, abbreviated Fru-2,6-P2, is a metabolite that allosterically affects the activity of the enzymes phosphofructokinase 1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1) to regulate glycolysis and gluconeogenesis. Fru-2,6-P2 itself is synthesized and broken down in either direction by the integrated bifunctional enzyme phosphofructokinase 2 (PFK-2/FBPase-2), which also contains a phosphatase domain and is also known as fructose-2,6-bisphosphatase. Whether the kinase and phosphatase domains of PFK-2/FBPase-2 are active or inactive depends on the phosphorylation state of the enzyme.
The Randle cycle, also known as the glucose fatty-acid cycle, is a metabolic process involving the competition of glucose and fatty acids for substrates. It is theorized to play a role in explaining type 2 diabetes and insulin resistance.
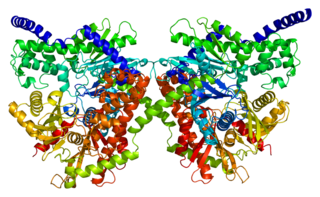
Hexokinase-1 (HK1) is an enzyme that in humans is encoded by the HK1 gene on chromosome 10. Hexokinases phosphorylate glucose to produce glucose-6-phosphate (G6P), the first step in most glucose metabolism pathways. This gene encodes a ubiquitous form of hexokinase which localizes to the outer membrane of mitochondria. Mutations in this gene have been associated with hemolytic anemia due to hexokinase deficiency. Alternative splicing of this gene results in five transcript variants which encode different isoforms, some of which are tissue-specific. Each isoform has a distinct N-terminus; the remainder of the protein is identical among all the isoforms. A sixth transcript variant has been described, but due to the presence of several stop codons, it is not thought to encode a protein. [provided by RefSeq, Apr 2009]

Hexokinase 2 also known as HK2 is an enzyme which in humans is encoded by the HK2 gene on chromosome 2. Hexokinases phosphorylate glucose to produce glucose-6-phosphate (G6P), the first step in most glucose metabolism pathways. This gene encodes hexokinase 2, the predominant form found in skeletal muscle. It localizes to the outer membrane of mitochondria. Expression of this gene is insulin-responsive, and studies in rat suggest that it is involved in the increased rate of glycolysis seen in rapidly growing cancer cells. [provided by RefSeq, Apr 2009]

Hexokinase 3 also known as HK3 is an enzyme which in humans is encoded by the HK3 gene on chromosome 5. Hexokinases phosphorylate glucose to produce glucose-6-phosphate (G6P), the first step in most glucose metabolism pathways. This gene encodes hexokinase 3. Similar to hexokinases 1 and 2, this allosteric enzyme is inhibited by its product glucose-6-phosphate. [provided by RefSeq, Apr 2009]
The insulin transduction pathway is a biochemical pathway by which insulin increases the uptake of glucose into fat and muscle cells and reduces the synthesis of glucose in the liver and hence is involved in maintaining glucose homeostasis. This pathway is also influenced by fed versus fasting states, stress levels, and a variety of other hormones.