Related Research Articles

Oxidative phosphorylation or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis.
A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN. Like all catalysts, they catalyze reverse as well as forward reactions, and in some cases this has physiological significance: for example, alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde in animals, but in yeast it catalyzes the production of ethanol from acetaldehyde.
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. Many of the enzymes in the electron transport chain are embedded within the membrane.
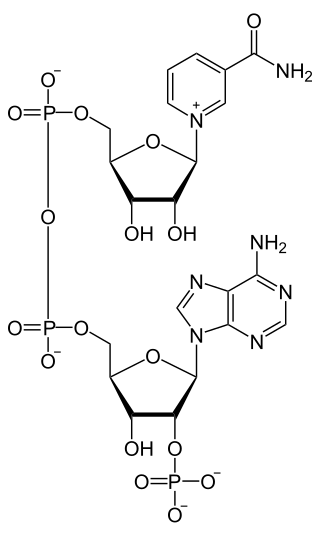
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form, whereas NADP+ is the oxidized form. NADP+ is used by all forms of cellular life.

Glycerol-3-phosphate dehydrogenase (GPDH) is an enzyme that catalyzes the reversible redox conversion of dihydroxyacetone phosphate to sn-glycerol 3-phosphate.
Formate dehydrogenases are a set of enzymes that catalyse the oxidation of formate to carbon dioxide, donating the electrons to a second substrate, such as NAD+ in formate:NAD+ oxidoreductase (EC 1.17.1.9) or to a cytochrome in formate:ferricytochrome-b1 oxidoreductase (EC 1.2.2.1). This family of enzymes has attracted attention as inspiration or guidance on methods for the carbon dioxide fixation, relevant to global warming.
In enzymology, a sorbitol-6-phosphate dehydrogenase (EC 1.1.1.140) is an enzyme that catalyzes the chemical reaction
In enzymology, an erythrose-4-phosphate dehydrogenase (EC 1.2.1.72) is an enzyme that catalyzes the chemical reaction

In enzymology, a D-xylulose reductase (EC 1.1.1.9) is an enzyme that is classified as an Oxidoreductase (EC 1) specifically acting on the CH-OH group of donors (EC 1.1.1) that uses NAD+ or NADP+ as an acceptor (EC 1.1.1.9). This enzyme participates in pentose and glucuronate interconversions; a set of metabolic pathways that involve converting pentose sugars and glucuronate into other compounds.
In enzymology, a 4-oxoproline reductase (EC 1.1.1.104) is an enzyme that catalyzes the chemical reaction
In enzymology, a mannitol-1-phosphate 5-dehydrogenase (EC 1.1.1.17) is an enzyme that catalyzes the chemical reaction
In enzymology, a mannuronate reductase (EC 1.1.1.131) is an enzyme that catalyzes the chemical reaction

In enzymology, a 3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35) is an enzyme that catalyzes the chemical reaction
In enzymology, a dihydrouracil dehydrogenase (NAD+) (EC 1.3.1.1) is an enzyme that catalyzes the chemical reaction
In enzymology, an enoyl-[acyl-carrier-protein] reductase (NADPH, A-specific) (EC 1.3.1.39) is an enzyme that catalyzes the chemical reaction
In enzymology, a glyceraldehyde-3-phosphate dehydrogenase (NAD(P)+) (EC 1.2.1.59) is an enzyme that catalyzes the chemical reaction
In enzymology, a glyceraldehyde-3-phosphate dehydrogenase (phosphorylating) (EC 1.2.1.12) is an enzyme that catalyzes the chemical reaction
In enzymology, a NAD(P)H dehydrogenase (quinone) (EC 1.6.5.2) is an enzyme that catalyzes the chemical reaction

In enzymology, a pyrroline-5-carboxylate reductase (EC 1.5.1.2) is an enzyme that catalyzes the chemical reaction

NADH:ubiquinone reductase (non-electrogenic) (EC 1.6.5.9, NDH-2, ubiquinone reductase, coenzyme Q reductase, dihydronicotinamide adenine dinucleotide-coenzyme Q reductase, DPNH-coenzyme Q reductase, DPNH-ubiquinone reductase, NADH-coenzyme Q oxidoreductase, NADH-coenzyme Q reductase, NADH-CoQ oxidoreductase, NADH-CoQ reductase) is an enzyme with systematic name NADH:ubiquinone oxidoreductase. This enzyme catalyses the following chemical reaction:
References
- ↑ Eric J. Toone (2006). Advances in Enzymology and Related Areas of Molecular Biology, Protein Evolution (Volume 75 ed.). Wiley-Interscience. ISBN 0471205036.
- ↑ Nicholas C. Price; Lewis Stevens (1999). Fundamentals of Enzymology: The Cell and Molecular Biology of Catalytic Proteins (Third ed.). USA: Oxford University Press. ISBN 019850229X.
- ↑ Superfamilies of single-pass transmembrane oxidoreductases in Membranome database