
Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. In humans, the DHFR enzyme is encoded by the DHFR gene. It is found in the q14.1 region of chromosome 5.

Platensimycin, a metabolite of Streptomyces platensis, is an antibiotic, which act by blocking enzymes.

Fatty acid synthase (FAS) is an enzyme that in humans is encoded by the FASN gene.

In molecular biology, Beta-ketoacyl-ACP synthase EC 2.3.1.41, is an enzyme involved in fatty acid synthesis. It typically uses malonyl-CoA as a carbon source to elongate ACP-bound acyl species, resulting in the formation of ACP-bound β-ketoacyl species such as acetoacetyl-ACP.

In enzymology, a 3-oxoacyl-[acyl-carrier-protein] reductase (EC 1.1.1.100) is an enzyme that catalyzes the chemical reaction

In enzymology, an acyl-CoA dehydrogenase (NADP+) (EC 1.3.1.8) is an enzyme that catalyzes the chemical reaction
In enzymology, an enoyl-[acyl-carrier-protein] reductase (NADPH, A-specific) (EC 1.3.1.39) is an enzyme that catalyzes the chemical reaction
In enzymology, an enoyl-[acyl-carrier-protein] reductase (NADPH, B-specific) (EC 1.3.1.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a trans-2-enoyl-CoA reductase (NADPH) (EC 1.3.1.38) is an enzyme that catalyzes the chemical reaction
In enzymology, a trans-2-decenoyl-[acyl-carrier protein] isomerase is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-hydroxypalmitoyl-[acyl-carrier-protein] dehydratase (EC 4.2.1.61) is an enzyme that catalyzes the chemical reaction
In enzymology, a crotonoyl-[acyl-carrier-protein] hydratase (EC 4.2.1.58) is an enzyme that catalyzes the chemical reaction
In enzymology, a [acyl-carrier-protein] S-malonyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a beta-ketoacyl-acyl-carrier-protein synthase II (EC 2.3.1.179) is an enzyme that catalyzes the chemical reaction

In enzymology, a β-ketoacyl-[acyl-carrier-protein] synthase III (EC 2.3.1.180) is an enzyme that catalyzes the chemical reaction

Fatty-acyl-CoA Synthase, or more commonly known as yeast fatty acid synthase, is an enzyme complex responsible for fatty acid biosynthesis, and is of Type I Fatty Acid Synthesis (FAS). Yeast fatty acid synthase plays a pivotal role in fatty acid synthesis. It is a 2.6 MDa barrel shaped complex and is composed of two, unique multi-functional subunits: alpha and beta. Together, the alpha and beta units are arranged in an α6β6 structure. The catalytic activities of this enzyme complex involves a coordination system of enzymatic reactions between the alpha and beta subunits. The enzyme complex therefore consists of six functional centers for fatty acid synthesis.
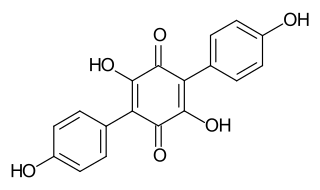
Atromentin is a natural chemical compound found in Agaricomycetes fungi in the orders Agaricales and Thelephorales. It can also be prepared by laboratory synthesis. Chemically, it is a polyphenol and a benzoquinone.

Omphalotus subilludens is a fungus species in the genus Omphalotus. The type collection was found by Murrill on July 26, 1944, in Gainesville, Florida. It has also been recorded from Texas.
Avadhesha Surolia is a glycobiologist at the Indian Institute of Science (IISc), Bangalore. He was born in Kishangarh, Rajasthan, India. Presently, he is an honorary professor at the Molecular Biophysics Unit, IISc and holds the Bhatnagar fellowship of the Council of Scientific and Industrial Research (CSIR). He is known for his work on lectin structure and interactions, orientation and dynamics of cell surface carbohydrate receptors and protein folding, diabetes, antimalarials and anti-cancer agents based on curcumin, flavonoids, etc. In addition, neuropathic pain, neurodegenerative disorders and the link between immunity and obsessive–compulsive disorder are areas of his current interest

Ketoacyl synthases (KSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. This reaction is a key step in the fatty acid synthesis cycle, as the resulting acyl chain is two carbon atoms longer than before. KSs exist as individual enzymes, as they do in type II fatty acid synthesis and type II polyketide synthesis, or as domains in large multidomain enzymes, such as type I fatty acid synthases (FASs) and polyketide synthases (PKSs). KSs are divided into five families: KS1, KS2, KS3, KS4, and KS5.












