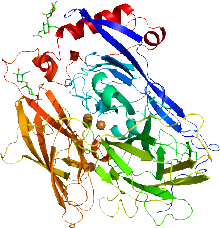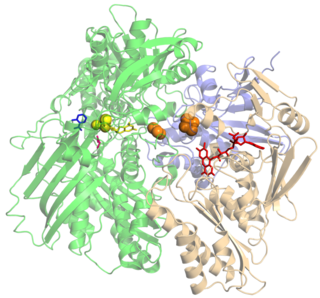
Xanthine oxidase is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans.
In biochemistry, an oxidase is an oxidoreductase (any enzyme that catalyzes a redox reaction) that uses dioxygen (O2) as the electron acceptor. In reactions involving donation of a hydrogen atom, oxygen is reduced to water (H2O) or hydrogen peroxide (H2O2). Some oxidation reactions, such as those involving monoamine oxidase or xanthine oxidase, typically do not involve free molecular oxygen.

The glucose oxidase enzyme also known as notatin is an oxidoreductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. This enzyme is produced by certain species of fungi and insects and displays antibacterial activity when oxygen and glucose are present.
Laccases are multicopper oxidases found in plants, fungi, and bacteria. Laccases oxidize a variety of phenolic substrates, performing one-electron oxidations, leading to crosslinking. For example, laccases play a role in the formation of lignin by promoting the oxidative coupling of monolignols, a family of naturally occurring phenols. Other laccases, such as those produced by the fungus Pleurotus ostreatus, play a role in the degradation of lignin, and can therefore be classed as lignin-modifying enzymes. Other laccases produced by fungi can facilitate the biosynthesis of melanin pigments. Laccases catalyze ring cleavage of aromatic compounds.
(S)-Tetrahydroberberine oxidase is an enzyme that catalyzes the final transformation in the biosynthesis of berberine, a quaternary benzylisoquinoline alkaloid of the protoberberine structural subgroup. This reaction pathway catalyzes the four-electron oxidation of (S)-tetrahydroberberine in the presence of oxygen to produce berberine and hydrogen peroxide as products.
In enzymology, a choline dehydrogenase is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acylhexosamine oxidase (EC 1.1.3.29) is an enzyme that catalyzes the chemical reaction
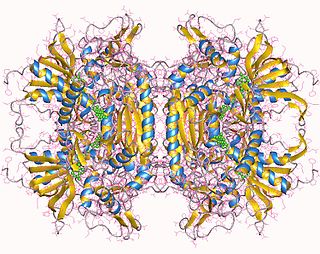
In enzymology, a pyranose oxidase (EC 1.1.3.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a secondary-alcohol oxidase (EC 1.1.3.18) is an enzyme that catalyzes the chemical reaction
Ascorbate 2,3-dioxygenase (EC 1.13.11.13) is an enzyme that catalyzes the chemical reaction
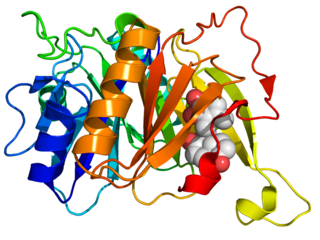
Biphenyl-2,3-diol 1,2-dioxygenase (EC 1.13.11.39) is an enzyme that catalyzes the chemical reaction
In enzymology, a lignostilbene alphabeta-dioxygenase (EC 1.13.11.43) is an enzyme that catalyzes the chemical reaction

Amine oxidase (copper-containing) (AOC) (EC 1.4.3.21 and EC 1.4.3.22; formerly EC 1.4.3.6) is a family of amine oxidase enzymes which includes both primary-amine oxidase and diamine oxidase; these enzymes catalyze the oxidation of a wide range of biogenic amines including many neurotransmitters, histamine and xenobiotic amines. They act as a disulphide-linked homodimer. They catalyse the oxidation of primary amines to aldehydes, with the subsequent release of ammonia and hydrogen peroxide, which requires one copper ion per subunit and topaquinone as cofactor:
In enzymology, a dimethylglycine oxidase (EC 1.5.3.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a glutathione oxidase (EC 1.8.3.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a L-glutamate oxidase (EC 1.4.3.11) is an enzyme that catalyzes the chemical reaction
In enzymology, a L-lysine 6-oxidase (EC 1.4.3.20) is an enzyme that catalyzes the chemical reaction
Nucleoside oxidase (EC 1.1.3.28) is an enzyme with systematic name nucleoside:oxygen 5'-oxidoreductase. This enzyme catalyses the following chemical reaction
Prosolanapyrone-II oxidase (EC 1.1.3.42, Sol5, SPS, solanapyrone synthase (bifunctional enzyme: prosolanapyrone II oxidase/prosolanapyrone III cycloisomerase), prosolanapyrone II oxidase) is an enzyme with systematic name prosolanapyrone-II:oxygen 3'-oxidoreductase. This enzyme catalyses the following chemical reaction
In chemistry, the oxygen reduction reaction refers to the reduction half reaction whereby O2 is reduced to water or hydrogen peroxide. In fuel cells, the reduction to water is preferred because the current is higher. The oxygen reduction reaction is well demonstrated and highly efficient in nature.