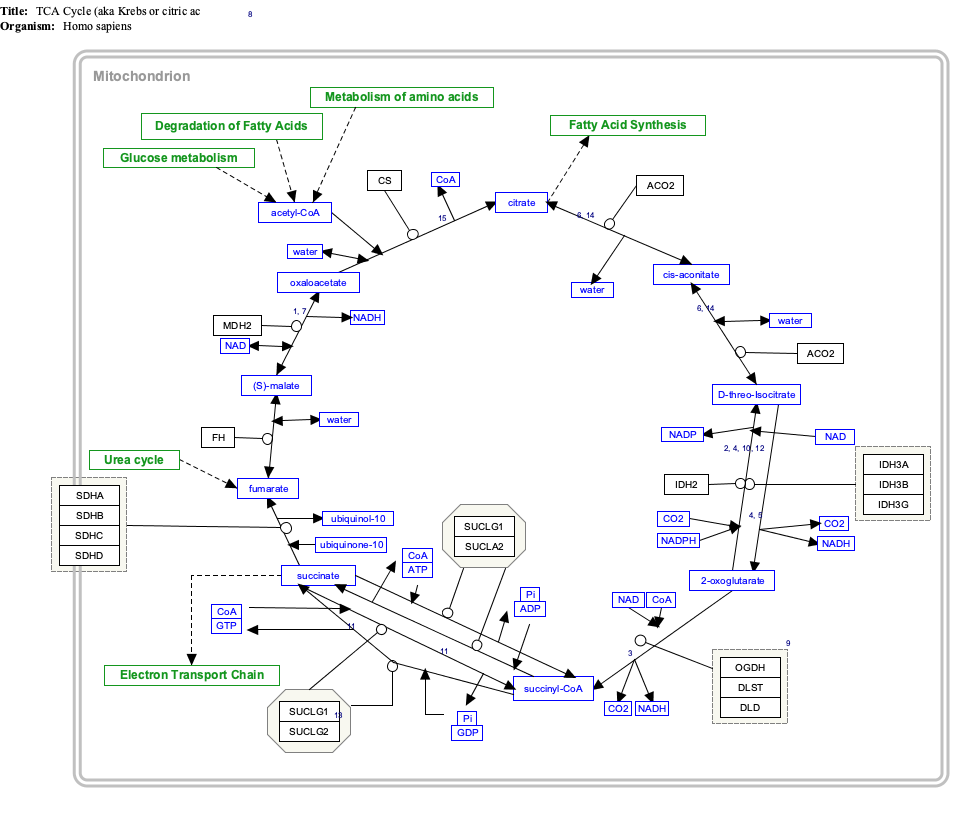
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. The electrons that are transferred from NADH and FADH2 to the ETC involves four multi-subunit large enzymes complexes and two mobile electron carriers. Many of the enzymes in the electron transport chain are embedded within the membrane.
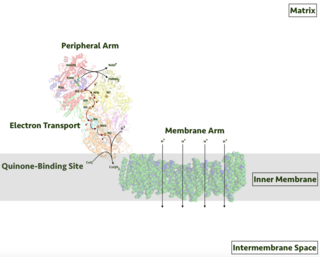
Respiratory complex I, EC 7.1.1.2 is the first large protein complex of the respiratory chains of many organisms from bacteria to humans. It catalyzes the transfer of electrons from NADH to coenzyme Q10 (CoQ10) and translocates protons across the inner mitochondrial membrane in eukaryotes or the plasma membrane of bacteria.

Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates in both the citric acid cycle and the electron transport chain. Histochemical analysis showing high succinate dehydrogenase in muscle demonstrates high mitochondrial content and high oxidative potential.

Succinate dehydrogenase [ubiquinone] cytochrome b small subunit, mitochondrial (CybS), also known as succinate dehydrogenase complex subunit D (SDHD), is a protein that in humans is encoded by the SDHD gene. Names previously used for SDHD were PGL and PGL1. Succinate dehydrogenase is an important enzyme in both the citric acid cycle and the electron transport chain. Hereditary PGL-PCC syndrome is caused by a parental imprint of the SDHD gene. Screening can begin by 6 years of age.

A paraganglioma is a rare neuroendocrine neoplasm that may develop at various body sites. When the same type of tumor is found in the adrenal gland, they are referred to as a pheochromocytoma. They are rare tumors, with an overall estimated incidence of 1/300,000. There is no test that determines benign from malignant tumors; long-term follow-up is therefore recommended for all individuals with paraganglioma.

Succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial (SDHB) also known as iron-sulfur subunit of complex II (Ip) is a protein that in humans is encoded by the SDHB gene.

Succinate dehydrogenase complex, subunit A, flavoprotein variant is a protein that in humans is encoded by the SDHA gene. This gene encodes a major catalytic subunit of succinate-ubiquinone oxidoreductase, a complex of the mitochondrial respiratory chain. The complex is composed of four nuclear-encoded subunits and is localized in the mitochondrial inner membrane. SDHA contains the FAD binding site where succinate is deprotonated and converted to fumarate. Mutations in this gene have been associated with a form of mitochondrial respiratory chain deficiency known as Leigh Syndrome. A pseudogene has been identified on chromosome 3q29. Alternatively spliced transcript variants encoding different isoforms have been found for this gene.

MT-ND6 is a gene of the mitochondrial genome coding for the NADH-ubiquinone oxidoreductase chain 6 protein (ND6). The ND6 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. Variations in the human MT-ND6 gene are associated with Leigh's syndrome, Leber's hereditary optic neuropathy (LHON) and dystonia.

MT-ND5 is a gene of the mitochondrial genome coding for the NADH-ubiquinone oxidoreductase chain 5 protein (ND5). The ND5 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. Variations in human MT-ND5 are associated with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) as well as some symptoms of Leigh's syndrome and Leber's hereditary optic neuropathy (LHON).

NADH dehydrogenase [ubiquinone] iron-sulfur protein 4, mitochondrial (NDUFS4) also known as NADH-ubiquinone oxidoreductase 18 kDa subunit is an enzyme that in humans is encoded by the NDUFS4 gene. This gene encodes a nuclear-encoded accessory subunit of the mitochondrial membrane respiratory chain NADH dehydrogenase. Complex I removes electrons from NADH and passes them to the electron acceptor ubiquinone. Mutations in this gene can cause mitochondrial complex I deficiencies such as Leigh syndrome.

NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 1 is a protein that in humans is encoded by the NDUFA1 gene. The NDUFA1 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. Mutations in the NDUFA1 gene are associated with mitochondrial Complex I deficiency.

NADH-ubiquinone oxidoreductase 75 kDa subunit, mitochondrial (NDUFS1) is an enzyme that in humans is encoded by the NDUFS1 gene. The encoded protein, NDUFS1, is the largest subunit of complex I, located on the inner mitochondrial membrane, and is important for mitochondrial oxidative phosphorylation. Mutations in this gene are associated with complex I deficiency.

NADH dehydrogenase [ubiquinone] iron-sulfur protein 7, mitochondrial, also knowns as NADH-ubiquinone oxidoreductase 20 kDa subunit, Complex I-20kD (CI-20kD), or PSST subunit is an enzyme that in humans is encoded by the NDUFS7 gene. The NDUFS7 protein is a subunit of NADH dehydrogenase (ubiquinone) also known as Complex I, which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain.

NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 6, also known as complex I-B17, is a protein that in humans is encoded by the NDUFB6 gene. NADH dehydrogenase (ubiquinone) 1 beta subcomplex subunit 6, is an accessory subunit of the NADH dehydrogenase (ubiquinone) complex, located in the mitochondrial inner membrane. It is also known as Complex I and is the largest of the five complexes of the electron transport chain.

NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 9 is an enzyme that in humans is encoded by the NDUFB9 gene. NADH dehydrogenase (ubiquinone) 1 beta subcomplex subunit 9 is an accessory subunit of the NADH dehydrogenase (ubiquinone) complex, located in the mitochondrial inner membrane. It is also known as Complex I and is the largest of the five complexes of the electron transport chain.

NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 11, mitochondrial is an enzyme that in humans is encoded by the NDUFB11 gene. NADH dehydrogenase (ubiquinone) 1 beta subcomplex subunit 11 is an accessory subunit of the NADH dehydrogenase (ubiquinone) complex, located in the mitochondrial inner membrane. It is also known as Complex I and is the largest of the five complexes of the electron transport chain. NDUFB11 mutations have been associated with linear skin defects with multiple congenital anomalies 3 and mitochondrial complex I deficiency.

Carney triad (CT) is characterized by the coexistence of three types of neoplasms, mainly in young women, including gastric gastrointestinal stromal tumor, pulmonary chondroma, and extra-adrenal paraganglioma. The underlying genetic defect remains elusive. CT is distinct from Carney complex, and the Carney-Stratakis syndrome.

Succinate dehydrogenase complex assembly factor 2, formerly known as SDH5 and also known as SDH assembly factor 2 or SDHAF2 is a protein that in humans is encoded by the SDHAF2 gene. This gene encodes a mitochondrial protein needed for the flavination of a succinate dehydrogenase complex subunit required for activity of the complex. Mutations in this gene are associated with pheochromocytoma and paraganglioma.

NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 11 is an enzyme that in humans is encoded by the NDUFA11 gene. The NDUFA11 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain Mutations in subunits of NADH dehydrogenase (ubiquinone), also known as Complex I, frequently lead to complex neurodegenerative diseases such as Leigh's syndrome. Mutations in this gene are associated with severe mitochondrial complex I deficiency.

Succinate dehydrogenase complex assembly factor 1 (SDHAF1), also known as LYR motif-containing protein 8 (LYRM8), is a protein that in humans is encoded by the SDHAF1, or LYRM8, gene. SDHAF1 is a chaperone protein involved in the assembly of the succinate dehydrogenase (SDH) complex. Mutations in this gene are associated with SDH-defective infantile leukoencephalopathy and mitochondrial complex II deficiency.