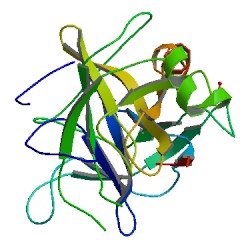
Chymotrypsin (EC 3.4.21.1, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenum, where it performs proteolysis, the breakdown of proteins and polypeptides. Chymotrypsin preferentially cleaves peptide amide bonds where the side chain of the amino acid N-terminal to the scissile amide bond (the P1 position) is a large hydrophobic amino acid (tyrosine, tryptophan, and phenylalanine). These amino acids contain an aromatic ring in their side chain that fits into a hydrophobic pocket (the S1 position) of the enzyme. It is activated in the presence of trypsin. The hydrophobic and shape complementarity between the peptide substrate P1 side chain and the enzyme S1 binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particularly those containing leucine at the P1 position.
Hydrolysis is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
In biochemistry, a ligase is an enzyme that can catalyze the joining (ligation) of two molecules by forming a new chemical bond. This is typically via hydrolysis of a small pendant chemical group on one of the molecules, typically resulting in the formation of new C-O, C-S, or C-N bonds. For example, DNA ligase can join two complementary fragments of nucleic acid by forming phosphodiester bonds, and repair single stranded breaks that arise in double stranded DNA during replication.

Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion.

A protease is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism, and cell signaling.
A glycosidic bond or glycosidic linkage is a type of ether bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.

In biochemistry, a nuclease is an enzyme capable of cleaving the phosphodiester bonds between nucleotides of nucleic acids. Nucleases variously effect single and double stranded breaks in their target molecules. In living organisms, they are essential machinery for many aspects of DNA repair. Defects in certain nucleases can cause genetic instability or immunodeficiency. Nucleases are also extensively used in molecular cloning.
An acid hydrolase is an enzyme that works best at acidic pHs. It is commonly located in lysosomes, which are acidic on the inside. Acid hydrolases may be nucleases, proteases, glycosidases, lipases, phosphatases, sulfatases and phospholipases and make up the approximately 50 degradative enzymes of the lysosome that break apart biological matter.
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure. The reverse reaction is also possible. For example, an enzyme that catalyzed this reaction would be a lyase:
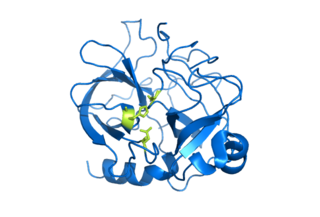
Serine proteases are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site. They are found ubiquitously in both eukaryotes and prokaryotes. Serine proteases fall into two broad categories based on their structure: chymotrypsin-like (trypsin-like) or subtilisin-like.

Digestive enzymes are a group of enzymes that break down polymeric macromolecules into their smaller building blocks, in order to facilitate their absorption into the cells of the body. Digestive enzymes are found in the digestive tracts of animals and in the tracts of carnivorous plants, where they aid in the digestion of food, as well as inside cells, especially in their lysosomes, where they function to maintain cellular survival. Digestive enzymes of diverse specificities are found in the saliva secreted by the salivary glands, in the secretions of cells lining the stomach, in the pancreatic juice secreted by pancreatic exocrine cells, and in the secretions of cells lining the small and large intestines.

A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.
In biochemistry, an esterase is a class of enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis.
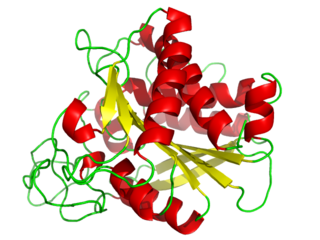
A carboxypeptidase is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed.

Aspartic proteases are a catalytic type of protease enzymes that use an activated water molecule bound to one or more aspartate residues for catalysis of their peptide substrates. In general, they have two highly conserved aspartates in the active site and are optimally active at acidic pH. Nearly all known aspartyl proteases are inhibited by pepstatin.
Serine hydrolases are one of the largest known enzyme classes comprising approximately ~200 enzymes or 1% of the genes in the human proteome. A defining characteristic of these enzymes is the presence of a particular serine at the active site, which is used for the hydrolysis of substrates. The hydrolysis of the ester or peptide bond proceeds in two steps. First, the acyl part of the substrate is transferred to the serine, making a new ester or amide bond and releasing the other part of the substrate is released. Later, in a slower step, the bond between the serine and the acyl group is hydrolyzed by water or hydroxide ion, regenerating free enzyme. Unlike other, non-catalytic, serines, the reactive serine of these hydrolases is typically activated by a proton relay involving a catalytic triad consisting of the serine, an acidic residue and a basic residue, although variations on this mechanism exist.
Lysosomal lipase is a form of lipase which functions intracellularly, in the lysosomes.
In enzymology, a glycosphingolipid deacylase is an enzyme that catalyzes a chemical reaction that cleaves gangliosides and neutral glycosphingolipids, releasing fatty acids to form the lyso-derivatives.
Lysine carboxypeptidase is an enzyme. This enzyme catalyses the following chemical reaction:









