Eosinophils, sometimes called eosinophiles or, less commonly, acidophils, are a variety of white blood cells and one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. Along with mast cells and basophils, they also control mechanisms associated with allergy and asthma. They are granulocytes that develop during hematopoiesis in the bone marrow before migrating into blood, after which they are terminally differentiated and do not multiply.

Eosinophilia is a condition in which the eosinophil count in the peripheral blood exceeds 5×108/L (500/μL). Hypereosinophilia is an elevation in an individual's circulating blood eosinophil count above 1.5 × 109/L (i.e. 1,500/μL). The hypereosinophilic syndrome is a sustained elevation in this count above 1.5 × 109/L (i.e. 1,500/μL) that is also associated with evidence of eosinophil-based tissue injury.

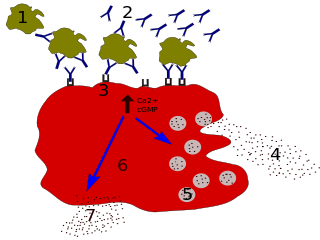
Basophils are a type of white blood cell. Basophils are the least common type of granulocyte, representing about 0.5% to 1% of circulating white blood cells. They are the largest type of granulocyte. They are responsible for inflammatory reactions during immune response, as well as in the formation of acute and chronic allergic diseases, including anaphylaxis, asthma, atopic dermatitis and hay fever. They also produce compounds that coordinate immune responses, including histamine and serotonin that induce inflammation, and heparin that prevents blood clotting, although there are less than that found in mast cell granules. Mast cells were once thought to be basophils that migrated from the blood into their resident tissues, but they are now known to be different types of cells.
Immunoglobulin E (IgE) is a type of antibody that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response against infection by certain parasitic worms, including Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is also utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum. IgE may have evolved as a defense to protect against venoms.

The interleukin 4 is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils. It is closely related and has functions similar to IL-13.

Interleukin 5 (IL-5) is an interleukin produced by type-2 T helper cells and mast cells.

Allergic inflammation is an important pathophysiological feature of several disabilities or medical conditions including allergic asthma, atopic dermatitis, allergic rhinitis and several ocular allergic diseases. Allergic reactions may generally be divided into two components; the early phase reaction, and the late phase reaction. While the contribution to the development of symptoms from each of the phases varies greatly between diseases, both are usually present and provide us a framework for understanding allergic disease.
Interleukin 33 (IL-33) is a protein that in humans is encoded by the IL33 gene.

Degranulation is a cellular process that releases antimicrobial, cytotoxic, or other molecules from secretory vesicles called granules found inside some cells. It is used by several different cells involved in the immune system, including granulocytes. It is also used by certain lymphocytes such as natural killer (NK) cells and cytotoxic T cells, whose main purpose is to destroy invading microorganisms.

The prostaglandin D2 receptor 1 (DP1), a G protein-coupled receptor encoded by the PTGDR1 gene (also termed PTGDR), is primarily a receptor for prostaglandin D2 (PGD2). The receptor is a member of the prostaglandin receptors belonging to the subfamily A14 of rhodopsin-like receptors. Activation of DP1 by PGD2 or other cognate receptor ligands is associated with a variety of physiological and pathological responses in animal models.

Pancreatic ribonuclease family is a superfamily of pyrimidine-specific endonucleases found in high quantity in the pancreas of certain mammals and of some reptiles.

Eosinophil-derived neurotoxin is an enzyme that in humans is encoded by the RNASE2 gene.
Creola bodies are a histopathologic finding indicative of asthma. Found in a patient's sputum, they are ciliated columnar cells sloughed from the bronchial mucosa of a patient with asthma. Other common findings in the sputum of asthma patients include Charcot-Leyden crystals, Curschmann's Spirals, and eosinophils.

Cysteinyl leukotriene receptor 1, also termed CYSLTR1, is a receptor for cysteinyl leukotrienes (LT). CYSLTR1, by binding these cysteinyl LTs contributes to mediating various allergic and hypersensitivity reactions in humans as well as models of the reactions in other animals.

Prostaglandin D2 receptor 2 (DP2 or CRTH2) is a human protein encoded by the PTGDR2 gene and GPR44. DP2 has also been designated as CD294 (cluster of differentiation 294). It is a member of the class of prostaglandin receptors which bind with and respond to various prostaglandins. DP2 along with prostaglandin DP1 receptor are receptors for prostglandin D2 (PGD2). Activation of DP2 by PGD2 or other cognate receptor ligands has been associated with certain physiological and pathological responses, particularly those associated with allergy and inflammation, in animal models and certain human diseases.

Cysteinyl leukotriene receptor 2, also termed CYSLTR2, is a receptor for cysteinyl leukotrienes (LT). CYSLTR2, by binding these cysteinyl LTs contributes to mediating various allergic and hypersensitivity reactions in humans. However, the first discovered receptor for these CsLTs, cysteinyl leukotriene receptor 1 (CysLTR1), appears to play the major role in mediating these reactions.

Peptidoglycan recognition protein 1, PGLYRP1, also known as TAG7, is an antibacterial and pro-inflammatory innate immunity protein that in humans is encoded by the PGLYRP1 gene.

Sialic acid-binding Ig-like lectin 8 is a protein that in humans is encoded by the SIGLEC8 gene. This gene is located on chromosome 19q13.4, about 330 kb downstream of the SIGLEC9 gene. Within the siglec family of transmembrane proteins, Siglec-8 belongs to the CD33-related siglec subfamily, a subfamily that has undergone rapid evolution.

5-Oxo-eicosatetraenoic acid is a nonclassic eicosanoid metabolite of arachidonic acid and the most potent naturally occurring member of the 5-HETE family of cell signaling agents. Like other cell signaling agents, 5-oxo-ETE is made by a cell and then feeds back to stimulate its parent cell and/or exits this cell to stimulate nearby cells. 5-Oxo-ETE can stimulate various cell types particularly human leukocytes but possesses its highest potency and power in stimulating the human eosinophil type of leukocyte. It is therefore suggested to be formed during and to be an important contributor to the formation and progression of eosinophil-based allergic reactions; it is also suggested that 5-oxo-ETE contributes to the development of inflammation, cancer cell growth, and other pathological and physiological events.
Lirentelimab is a humanized nonfucosylated monoclonal antibody that targets sialic acid-binding Ig-like lectin 8 (SIGLEC8). In a randomized clinical trial, lirentelimab was found to improve eosinophil counts and symptoms in individuals with eosinophilic gastritis and duodenitis. Adverse reactions include infusion reactions, which are mild to moderate and typically occur following the first infusion.