
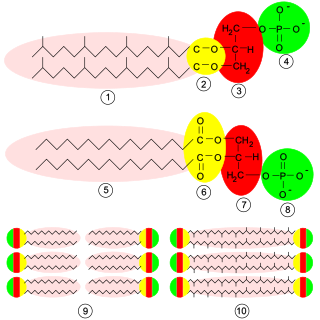
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue. Marine phospholipids typically have omega-3 fatty acids EPA and DHA integrated as part of the phospholipid molecule. The phosphate group can be modified with simple organic molecules such as choline, ethanolamine or serine.

A phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophilic substances. Acids trigger the release of bound calcium from cellular stores and the consequent increase in free cytosolic Ca2+, an essential step in calcium signaling to regulate intracellular processes. There are four major classes, termed A, B, C, and D, which are distinguished by the type of reaction which they catalyze:

Phosphatidylcholines (PC) are a class of phospholipids that incorporate choline as a headgroup. They are a major component of biological membranes and can be easily obtained from a variety of readily available sources, such as egg yolk or soybeans, from which they are mechanically or chemically extracted using hexane. They are also a member of the lecithin group of yellow-brownish fatty substances occurring in animal and plant tissues. Dipalmitoylphosphatidylcholine (lecithin) is a major component of the pulmonary surfactant, and is often used in the lecithin–sphingomyelin ratio to calculate fetal lung maturity. While phosphatidylcholines are found in all plant and animal cells, they are absent in the membranes of most bacteria, including Escherichia coli. Purified phosphatidylcholine is produced commercially.

The enzyme phospholipase A2 (EC 3.1.1.4, PLA2, systematic name phosphatidylcholine 2-acylhydrolase) catalyse the cleavage of fatty acids in position 2 of phospholipids, hydrolyzing the bond between the second fatty acid “tail” and the glycerol molecule:
Glycerophospholipids or phosphoglycerides are glycerol-based phospholipids. They are the main component of biological membranes. Two major classes are known: those for bacteria and eukaryotes and a separate family for archaea.
Phospholipase D (EC 3.1.4.4, lipophosphodiesterase II, lecithinase D, choline phosphatase, PLD; systematic name phosphatidylcholine phosphatidohydrolase) is an enzyme of the phospholipase superfamily that catalyses the following reaction
Taipoxin is a potent myo- and neurotoxin that was isolated from the venom of the coastal taipan Oxyuranus scutellatus or also known as the common taipan. Taipoxin like many other pre-synaptic neurotoxins are phospholipase A2 (PLA2) toxins, which inhibit/complete block the release of the motor transmitter acetylcholine and lead to death by paralysis of the respiratory muscles (asphyxia). It is the most lethal neurotoxin isolated from any snake venom to date.

Phosphatidylethanolamine (PE) is a class of phospholipids found in biological membranes. They are synthesized by the addition of cytidine diphosphate-ethanolamine to diglycerides, releasing cytidine monophosphate. S-Adenosyl methionine can subsequently methylate the amine of phosphatidylethanolamines to yield phosphatidylcholines.
The lysophospholipid receptor (LPL-R) group are members of the G protein-coupled receptor family of integral membrane proteins that are important for lipid signaling. In humans, there are eleven LPL receptors, each encoded by a separate gene. These LPL receptor genes are also sometimes referred to as "Edg".
The enzyme lysophospholipase (EC 3.1.1.5) catalyzes the reaction

The enzyme phosphatidate phosphatase (PAP, EC 3.1.3.4) is a key regulatory enzyme in lipid metabolism, catalyzing the conversion of phosphatidate to diacylglycerol:

Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role in eukaryotic cell physiology, in particular signal transduction pathways. Phospholipase C's role in signal transduction is its cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. Activators of each PLC vary, but typically include heterotrimeric G protein subunits, protein tyrosine kinases, small G proteins, Ca2+, and phospholipids.

85 kDa calcium-independent phospholipase A2, also known as 85/88 kDa calcium-independent phospholipase A2, Group VI phospholipase A2, Intracellular membrane-associated calcium-independent phospholipase A2 beta, or Patatin-like phospholipase domain-containing protein 9 is an enzyme that in humans is encoded by the PLA2G6 gene.

Calcium-dependent phospholipase A2 is an enzyme that in humans is encoded by the PLA2G5 gene.

Platelet-activating factor acetylhydrolase 2, cytoplasmic is an enzyme that in humans is encoded by the PAFAH2 gene. It is one of several PAF acetylhydrolases.
N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) is an enzyme that catalyzes the release of N-acylethanolamine (NAE) from N-acyl-phosphatidylethanolamine (NAPE). This is a major part of the process that converts ordinary lipids into chemical signals like anandamide and oleoylethanolamine. In humans, the NAPE-PLD protein is encoded by the NAPEPLD gene.
Lipase is a family of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; however, these are usually treated separately from "conventional" lipases. Unlike esterases, which function in water, lipases "are activated only when adsorbed to an oil–water interface". Lipases perform essential roles in digestion, transport and processing of dietary lipids in most, if not all, organisms.

Lysophosphatidylcholines, also called lysolecithins, are a class of chemical compounds which are derived from phosphatidylcholines.

2-acyl-sn-glycero-3-phosphocholines are a class of phospholipids that are intermediates in the metabolism of lipids. Because they result from the hydrolysis of an acyl group from the sn-1 position of phosphatidylcholine, they are also called 1-lysophosphatidylcholine. The synthesis of phosphatidylcholines with specific fatty acids occurs through the synthesis of 1-lysoPC. The formation of various other lipids generates 1-lysoPC as a by-product.

Lysophospholipid acyltransferase 7 also known as membrane-bound O-acyltransferase domain-containing protein 7 (MBOAT7) is an enzyme that in humans is encoded by the MBOAT7 gene. It is homologous to other membrane-bound O-acyltransferases.














