
A phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophilic substances. There are four major classes, termed A, B, C, and D, which are distinguished by the type of reaction which they catalyze:

Arachidonic acid is a polyunsaturated omega−6 fatty acid 20:4(ω−6), or 20:4(5,8,11,14). If its precursors or diet contains linoleic acid it is formed by biosynthesis and can be deposited in animal fats. It is a precursor in the formation of leukotrienes, prostaglandins, and thromboxanes.

The enzyme phospholipase A2 (EC 3.1.1.4, PLA2, systematic name phosphatidylcholine 2-acylhydrolase) catalyses the cleavage of fatty acids in position 2 of phospholipids, hydrolyzing the bond between the second fatty acid "tail" and the glycerol molecule:
Taipoxin is a potent myo- and neurotoxin that was isolated from the venom of the coastal taipan Oxyuranus scutellatus or also known as the common taipan. Taipoxin like many other pre-synaptic neurotoxins are phospholipase A2 (PLA2) toxins, which inhibit/complete block the release of the motor transmitter acetylcholine and lead to death by paralysis of the respiratory muscles (asphyxia). It is the most lethal neurotoxin isolated from any snake venom to date.

Phospholipase A1 (EC 3.1.1.32; systematic name: phosphatidylcholine 1-acylhydrolase) encoded by the PLA1A gene is a phospholipase enzyme which removes the 1-acyl group:

Infantile neuroaxonal dystrophy (INAD) is a rare pervasive developmental disorder that primarily affects the nervous system. Individuals with infantile neuroaxonal dystrophy typically do not have any symptoms at birth, but between the ages of about 6 and 18 months they begin to experience delays in acquiring new motor and intellectual skills, such as crawling or beginning to speak. Eventually they lose previously acquired skills.

Cytosolic phospholipase A2 is an enzyme that in humans is encoded by the PLA2G4A gene.

Phospholipase A2, group 1B is an enzyme that in humans is encoded by the PLA2G1B gene.

85 kDa calcium-independent phospholipase A2, also known as 85/88 kDa calcium-independent phospholipase A2, Group VI phospholipase A2, Intracellular membrane-associated calcium-independent phospholipase A2 beta, or Patatin-like phospholipase domain-containing protein 9 is an enzyme that in humans is encoded by the PLA2G6 gene.

Cytosolic phospholipase A2 gamma is an enzyme that in humans is encoded by the PLA2G4C gene.

Phospholipase A2, group IVB (cytosolic) is an enzyme that in humans is encoded by the PLA2G4B gene.

Group XVI phospholipase A2 also commonly known as adipocyte phospholipase A2 (AdPLA) is an enzyme that in humans is encoded by the PLA2G16 gene. This enzyme has also been identified as PLA2G16, HRASLS3, HREV107, HREV107-3, MGC118754 or H-REV107-1 from studies on class II tumor suppression but not on its enzymatic properties. AdPLA is encoded by a 1.3 kilobase AdPLA messenger RNA and is an 18 kDa protein. It belongs to a superfamily of phospholipase A2 (PLA2) enzymes and is found primarily in adipose tissue. AdPLA regulates adipocyte lipolysis and release of fatty acids through a G-protein coupled pathway involving prostaglandin and EP3. It has also been reported to play a crucial role in the development of obesity in mouse models.

Darapladib is an inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2) that is in development as a drug for treatment of atherosclerosis.

Lipoprotein-associated phospholipase A2 (Lp-PLA2) also known as platelet-activating factor acetylhydrolase (PAF-AH) is a phospholipase A2 enzyme that in humans is encoded by the PLA2G7 gene. Lp-PLA2 is a 45-kDa protein of 441 amino acids. It is one of several PAF acetylhydrolases.
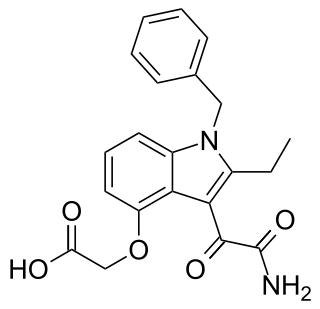
Varespladib is an inhibitor of the IIa, V, and X isoforms of secretory phospholipase A2 (sPLA2). The molecule acts as an anti-inflammatory agent by disrupting the first step of the arachidonic acid pathway of inflammation. From 2006 to 2012, varespladib was under active investigation by Anthera Pharmaceuticals as a potential therapy for several inflammatory diseases, including acute coronary syndrome and acute chest syndrome. The trial was halted in March 2012 due to inadequate efficacy. The selective sPLA2 inhibitor varespladib (IC50 value 0.009 μM in chromogenic assay, mole fraction 7.3X10-6) was studied in the VISTA-16 randomized clinical trial (clinicaltrials.gov Identifier: NCT01130246) and the results were published in 2014. The sPLA2 inhibition by varespladib in this setting seemed to be potentially harmful, and thus not a useful strategy for reducing adverse cardiovascular outcomes from acute coronary syndrome. Since 2016, scientific research has focused on the use of Varespladib as an inhibitor of snake venom toxins using various types of in vitro and in vivo models. Varespladib showed a significant inhibitory effect to snake venom PLA2 which makes it a potential first-line drug candidate in snakebite envenomation therapy. In 2019, the U.S. Food and Drug Administration (FDA) granted varespladib orphan drug status for its potential to treat snakebite.
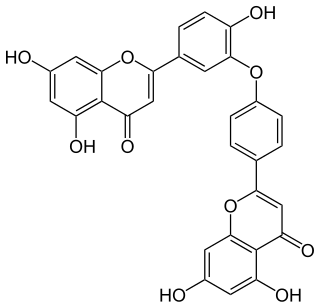
Ochnaflavone, a secondary plant secondary metabolite of the Biflavonoid family, has been widely investigated in past decades due to its unique ability to mediate biological activities, such as inhibition of phospholipase A2 and lymphocyte proliferation. It was first isolated from Ochna squarrosa Linn, a member of Ochnaceae family, in 1973.
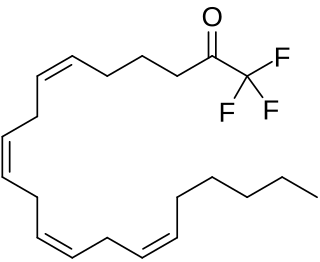
Arachidonyl trifluoromethyl ketone (ATK) is an analog of arachidonic acid. that inhibits some isoforms of the enzyme phospholipase A2. Specifically it inhibits the 85 kDa cystolic PLA2 (cPLA2).
Crotoxin (CTX) is the main toxic compound in the snake venom of the South American rattlesnake, Crotalus durissus terrificus. Crotoxin is a heterodimeric beta-neurotoxin, composed of an acidic, non-toxic and non-enzymatic subunit (CA), and a basic, weakly toxic, phospholipase A2 protein (CB). This neurotoxin causes paralysis by both pre- and postsynaptic blocking of acetylcholine signalling.
MiDCA1, short for Micrurus dumerili carinicauda 1, is a β-neurotoxin primarily affecting presynaptic synapses, where it interferes with the release of neurotransmitters by inhibiting potassium (K+) channels. This toxin belongs to the phospholipase A2 (PLA2) family but distinguishes itself by existing as a monomer, unlike some other PLA2 toxins. It occurs naturally in the venom of the coral snake Micrurus dumerili carinicauda.















