
Ribonuclease H is a family of non-sequence-specific endonuclease enzymes that catalyze the cleavage of RNA in an RNA/DNA substrate via a hydrolytic mechanism. Members of the RNase H family can be found in nearly all organisms, from bacteria to archaea to eukaryotes.

Angiogenin (ANG) also known as ribonuclease 5 is a small 123 amino acid protein that in humans is encoded by the ANG gene. Angiogenin is a potent stimulator of new blood vessels through the process of angiogenesis. Ang hydrolyzes cellular RNA, resulting in modulated levels of protein synthesis and interacts with DNA causing a promoter-like increase in the expression of rRNA. Ang is associated with cancer and neurological disease through angiogenesis and through activating gene expression that suppresses apoptosis.

Cathepsin C (CTSC) also known as dipeptidyl peptidase I (DPP-I) is a lysosomal exo-cysteine protease belonging to the peptidase C1 protein family, a subgroup of the cysteine cathepsins. In humans, it is encoded by the CTSC gene.

Ribonuclease pancreatic is an enzyme that in humans is encoded by the RNASE1 gene.

A 2-oxoisovalerate dehydrogenase subunit alpha, mitochondrial is an enzyme that in humans is encoded by the BCKDHA gene.

Three prime repair exonuclease 1 is an enzyme that in humans is encoded by the TREX1 gene.

Ribonuclease P protein subunit p20 is an enzyme that in humans is encoded by the POP7 gene.

Ribonuclease P protein subunit p40 is an enzyme that in humans is encoded by the RPP40 gene.

Ribonucleases P/MRP protein subunit POP1 is a protein that in humans is encoded by the POP1 gene.

Ribonuclease P protein subunit p30 is an enzyme that in humans is encoded by the RPP30 gene.

Ribonuclease P protein subunit p38 is an enzyme that in humans is encoded by the RPP38 gene.

Ribonuclease P protein subunit p14 is an enzyme that in humans is encoded by the RPP14 gene.

SAM domain and HD domain-containing protein 1 is a protein that in humans is encoded by the SAMHD1 gene. SAMHD1 is a cellular enzyme, responsible for blocking replication of HIV in dendritic cells, macrophages, monocytes and resting CD4+ T lymphocytes. It is an enzyme that exhibits phosphohydrolase activity, converting deoxynucleoside triphosphates (dNTPs) to inorganic phosphate (iPPP) and a 2'-deoxynucleoside (i.e. deoxynucleosides without a phosphate group). In doing so, SAMHD1 depletes the pool of dNTPs available to a reverse transcriptase for viral cDNA synthesis and thus prevents viral replication. SAMHD1 has also shown nuclease activity. Although a ribonuclease activity was described to be required for HIV-1 restriction, recent data confirmed that SAMHD1-mediated HIV-1 restriction in cells does not involve ribonuclease activity.

Dynein heavy chain 11, axonemal is a protein that in humans is encoded by the DNAH11 gene.

Ribonuclease H1 also known as RNase H1 is an enzyme that in humans is encoded by the RNASEH1 gene. The RNase H1 is a non-specific endonuclease and catalyzes the cleavage of RNA via a hydrolytic mechanism.

Ribonuclease H2 subunit A, also known as RNase H2 subunit A, is an enzyme that in humans is encoded by the RNASEH2A gene.
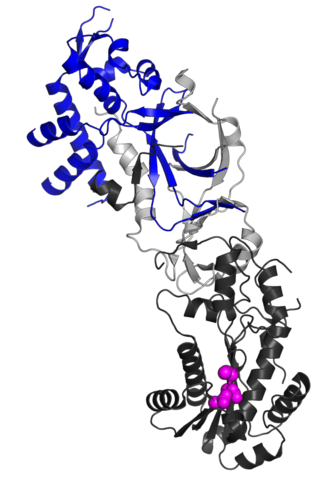
Ribonuclease H2, subunit B is a protein that in humans is encoded by the RNASEH2B gene. RNase H2 is composed of a single catalytic subunit (A) and two non-catalytic subunits, and degrades the RNA of RNA:DNA hybrids. The non-catalytic B subunit of RNase H2 is thought to play a role in DNA replication.

Mitochondrial ribosomal protein L3 is a protein that in humans is encoded by the MRPL3 gene.

ATP-binding cassette sub-family E member 1 (ABCE1) also known as RNase L inhibitor (RLI) is an enzyme that in humans is encoded by the ABCE1 gene.

Aicardi–Goutières syndrome (AGS), which is completely distinct from the similarly named Aicardi syndrome, is a rare, usually early onset childhood, inflammatory disorder most typically affecting the brain and the skin. The majority of affected individuals experience significant intellectual and physical problems, although this is not always the case. The clinical features of AGS can mimic those of in utero acquired infection, and some characteristics of the condition also overlap with the autoimmune disease systemic lupus erythematosus (SLE). Following an original description of eight cases in 1984, the condition was first referred to as 'Aicardi–Goutières syndrome' (AGS) in 1992, and the first international meeting on AGS was held in Pavia, Italy, in 2001.




















