A phosphodiesterase inhibitor is a drug that blocks one or more of the five subtypes of the enzyme phosphodiesterase (PDE), thereby preventing the inactivation of the intracellular second messengers, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) by the respective PDE subtype(s). The ubiquitous presence of this enzyme means that non-specific inhibitors have a wide range of actions, the actions in the heart, and lungs being some of the first to find a therapeutic use.

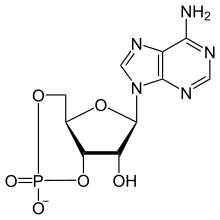
A cyclic nucleotide (cNMP) is a single-phosphate nucleotide with a cyclic bond arrangement between the sugar and phosphate groups. Like other nucleotides, cyclic nucleotides are composed of three functional groups: a sugar, a nitrogenous base, and a single phosphate group. As can be seen in the cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) images, the 'cyclic' portion consists of two bonds between the phosphate group and the 3' and 5' hydroxyl groups of the sugar, very often a ribose.

Tadalafil, sold under the brand name Cialis among others, is a medication used to treat erectile dysfunction, benign prostatic hyperplasia, and pulmonary arterial hypertension. It is taken by mouth. Onset is typically within half an hour and the duration is up to 36 hours.

Cyclic guanosine monophosphate (cGMP) is a cyclic nucleotide derived from guanosine triphosphate (GTP). cGMP acts as a second messenger much like cyclic AMP. Its most likely mechanism of action is activation of intracellular protein kinases in response to the binding of membrane-impermeable peptide hormones to the external cell surface. Through protein kinases activation, cGMP can relax smooth muscle. cGMP concentration in urine can be measured for kidney function and diabetes detection.

A phosphodiesterase type 5 inhibitor is a vasodilating drug that works by blocking the degradative action of cGMP-specific phosphodiesterase type 5 (PDE5) on cyclic GMP in the smooth muscle cells lining the blood vessels supplying various tissues. These drugs dilate the corpora cavernosa of the penis, facilitating erection with sexual stimulation, and are used in the treatment of erectile dysfunction (ED). Sildenafil was the first effective oral treatment available for ED. Because PDE5 is also present in the smooth muscle of the walls of the arterioles within the lungs, two PDE5 inhibitors, sildenafil and tadalafil, are FDA-approved for the treatment of pulmonary hypertension. As of 2019, the wider cardiovascular benefits of PDE5 inhibitors are being appreciated.

Cyclic guanosine monophosphate-specific phosphodiesterase type 5 is an enzyme from the phosphodiesterase class. It is found in various tissues, most prominently the corpus cavernosum and the retina. It has also been recently discovered to play a vital role in the cardiovascular system.

3′,5′-cyclic-nucleotide phosphodiesterases (EC 3.1.4.17) are a family of phosphodiesterases. Generally, these enzymes hydrolyze a nucleoside 3′,5′-cyclic phosphate to a nucleoside 5′-phosphate:

PDE3 is a phosphodiesterase. The PDEs belong to at least eleven related gene families, which are different in their primary structure, substrate affinity, responses to effectors, and regulation mechanism. Most of the PDE families are composed of more than one gene. PDE3 is clinically significant because of its role in regulating heart muscle, vascular smooth muscle and platelet aggregation. PDE3 inhibitors have been developed as pharmaceuticals, but their use is limited by arrhythmic effects and they can increase mortality in some applications.
Phosphodiesterase 1, PDE1, EC 3.1.4.1, systematic name oligonucleotide 5′-nucleotidohydrolase) is a phosphodiesterase enzyme also known as calcium- and calmodulin-dependent phosphodiesterase. It is one of the 11 families of phosphodiesterase (PDE1-PDE11). Phosphodiesterase 1 has three subtypes, PDE1A, PDE1B and PDE1C which divide further into various isoforms. The various isoforms exhibit different affinities for cAMP and cGMP.

The PDE2 enzyme is one of 21 different phosphodiesterases (PDE) found in mammals. These different PDEs can be subdivided to 11 families. The different PDEs of the same family are functionally related despite the fact that their amino acid sequences show considerable divergence. The PDEs have different substrate specificities. Some are cAMP selective hydrolases, others are cGMP selective hydrolases and the rest can hydrolyse both cAMP and cGMP.
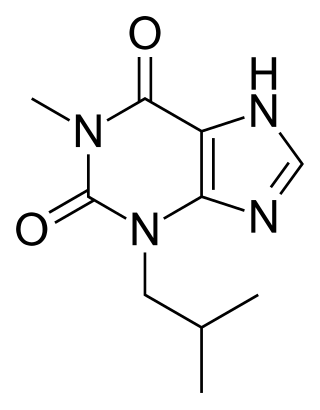
IBMX (3-isobutyl-1-methylxanthine), like other methylxanthine derivatives, is both a:
- competitive non-selective phosphodiesterase inhibitor which raises intracellular cAMP, activates PKA, inhibits TNFα and leukotriene synthesis, and reduces inflammation and innate immunity, and
- nonselective adenosine receptor antagonist.

Avanafil is a PDE5 inhibitor approved for erectile dysfunction by the FDA on April 27, 2012 and by EMA on June 21, 2013. Avanafil is sold under the brand names Stendra and Spedra. It was invented at Mitsubishi Tanabe Pharma, formerly known as Tanabe Seiyaku Co., and licensed to Vivus Inc., which partnered with Menarini Group to commercialise Spedra in over forty European countries, Australia, and New Zealand. Metuchen Pharmaceuticals obtained exclusive rights within the United States.
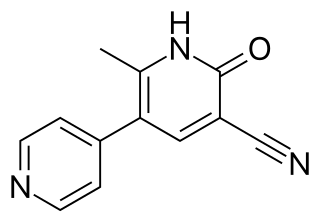
A PDE3 inhibitor is a drug which inhibits the action of the phosphodiesterase enzyme PDE3. They are used for the therapy of acute heart failure and cardiogenic shock.

cAMP-specific 3',5'-cyclic phosphodiesterase 4A is an enzyme that in humans is encoded by the PDE4A gene.

cAMP-specific 3',5'-cyclic phosphodiesterase 4B is an enzyme that in humans is encoded by the PDE4B gene.

Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A is an enzyme that in humans is encoded by the PDE11A gene.

cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A is an enzyme that in humans is encoded by the PDE10A gene.

Piclamilast, is a selective PDE4 inhibitor. It is comparable to other PDE4 inhibitors for its anti-inflammatory effects. It has been investigated for its applications to the treatment of conditions such as chronic obstructive pulmonary disease, bronchopulmonary dysplasia and asthma. It is a second generation compound that exhibits structural functionalities of the PDE4 inhibitors cilomilast and roflumilast. The structure for piclamilast was first elucidated in a 1995 European patent application. The earliest mention of the name "piclamilast" was used in a 1997 publication.
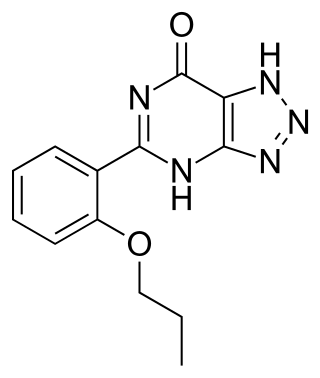
Zaprinast was an unsuccessful clinical drug candidate that was a precursor to the chemically related PDE5 inhibitors, such as sildenafil (Viagra), which successfully reached the market. It is a phosphodiesterase inhibitor, selective for the subtypes PDE5, PDE6, PDE9 and PDE11. IC50 values are 0.76, 0.15, 29.0, and 12.0 μM, respectively.
Phosphodiesterases (PDEs) are a superfamily of enzymes. This superfamily is further classified into 11 families, PDE1 - PDE11, on the basis of regulatory properties, amino acid sequences, substrate specificities, pharmacological properties and tissue distribution. Their function is to degrade intracellular second messengers such as cyclic adenine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) which leads to several biological processes like effect on intracellular calcium level by the Ca2+ pathway.