
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion.

Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsinogen proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized.

Lysine is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group, an α-carboxylic acid group, and a side chain (CH2)4NH2, and so it is classified as a basic, charged, aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the S configuration.

Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the l-arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid.

Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises the concentration of glucose and fatty acids in the bloodstream and is considered to be the main catabolic hormone of the body. It is also used as a medication to treat a number of health conditions. Its effect is opposite to that of insulin, which lowers extracellular glucose. It is produced from proglucagon, encoded by the GCG gene.
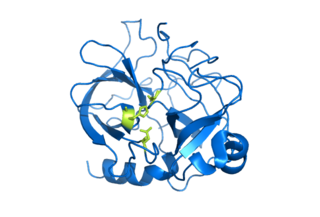
Serine proteases are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site. They are found ubiquitously in both eukaryotes and prokaryotes. Serine proteases fall into two broad categories based on their structure: chymotrypsin-like (trypsin-like) or subtilisin-like.
Trypsinogen is the precursor form of trypsin, a digestive enzyme. It is produced by the pancreas and found in pancreatic juice, along with amylase, lipase, and chymotrypsinogen. It is cleaved to its active form, trypsin, by enteropeptidase, which is found in the intestinal mucosa. Once activated, the trypsin can cleave more trypsinogen into trypsin, a process called autoactivation. Trypsin cleaves the peptide bond on the carboxyl side of basic amino acids such as arginine and lysine.

Amylin, or islet amyloid polypeptide (IAPP), is a 37-residue peptide hormone. It is co-secreted with insulin from the pancreatic β-cells in the ratio of approximately 100:1 (insulin:amylin). Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.
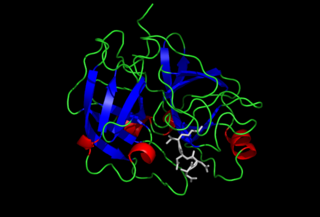
Enteropeptidase is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals. Enteropeptidase converts trypsinogen into its active form trypsin, resulting in the subsequent activation of pancreatic digestive enzymes. Absence of enteropeptidase results in intestinal digestion impairment.
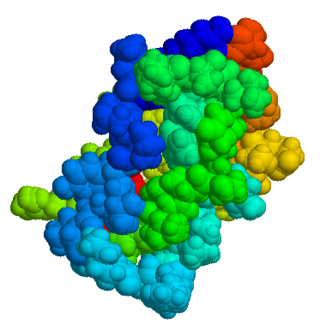
The drug aprotinin, is a small protein bovine pancreatic trypsin inhibitor (BPTI), or basic trypsin inhibitor of bovine pancreas, which is an antifibrinolytic molecule that inhibits trypsin and related proteolytic enzymes. Under the trade name Trasylol, aprotinin was used as a medication administered by injection to reduce bleeding during complex surgery, such as heart and liver surgery. Its main effect is the slowing down of fibrinolysis, the process that leads to the breakdown of blood clots. The aim in its use was to decrease the need for blood transfusions during surgery, as well as end-organ damage due to hypotension as a result of marked blood loss. The drug was temporarily withdrawn worldwide in 2007 after studies suggested that its use increased the risk of complications or death; this was confirmed by follow-up studies. Trasylol sales were suspended in May 2008, except for very restricted research use. In February 2012 the European Medicines Agency (EMA) scientific committee reverted its previous standpoint regarding aprotinin, and has recommended that the suspension be lifted. Nordic became distributor of aprotinin in 2012.
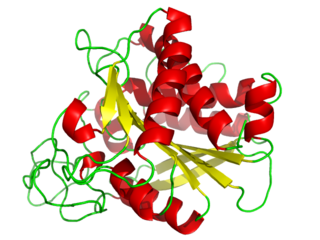
A carboxypeptidase is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed.
Protein metabolism denotes the various biochemical processes responsible for the synthesis of proteins and amino acids (anabolism), and the breakdown of proteins by catabolism.

Carboxypeptidase E (CPE), also known as carboxypeptidase H (CPH) and enkephalin convertase, is an enzyme that in humans is encoded by the CPE gene. This enzyme catalyzes the release of C-terminal arginine or lysine residues from polypeptides.

Carboxypeptidase A usually refers to the pancreatic exopeptidase that hydrolyzes peptide bonds of C-terminal residues with aromatic or aliphatic side-chains. Most scientists in the field now refer to this enzyme as CPA1, and to a related pancreatic carboxypeptidase as CPA2.
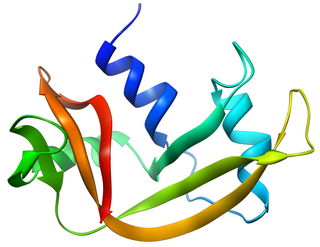
Pancreatic ribonuclease family is a superfamily of pyrimidine-specific endonucleases found in high quantity in the pancreas of certain mammals and of some reptiles.

The enzyme Acid-Induced Arginine Decarboxylase (AdiA), also commonly referred to as arginine decarboxylase, catalyzes the conversion of L-arginine into agmatine and carbon dioxide. The process consumes a proton in the decarboxylation and employs a pyridoxal-5'-phosphate (PLP) cofactor, similar to other enzymes involved in amino acid metabolism, such as ornithine decarboxylase and glutamine decarboxylase. It is found in bacteria and virus, though most research has so far focused on forms of the enzyme in bacteria. During the AdiA catalyzed decarboxylation of arginine, the necessary proton is consumed from the cell cytoplasm which helps to prevent the over-accumulation of protons inside the cell and serves to increase the intracellular pH. Arginine decarboxylase is part of an enzymatic system in Escherichia coli, Salmonella Typhimurium, and methane-producing bacteria Methanococcus jannaschii that makes these organisms acid resistant and allows them to survive under highly acidic medium.
Lysine carboxypeptidase is an enzyme. This enzyme catalyses the following chemical reaction:
Carboxypeptidase D can refer to one of several enzymes. A family of serine carboxypeptidases includes is an enzyme. This enzyme has an optimal pH of 4.5-6.0, is inhibited by diisopropyl fluorophosphate, and catalyses the following chemical reaction
Protein methylation is a type of post-translational modification featuring the addition of methyl groups to proteins. It can occur on the nitrogen-containing side-chains of arginine and lysine, but also at the amino- and carboxy-termini of a number of different proteins. In biology, methyltransferases catalyze the methylation process, activated primarily by S-adenosylmethionine. Protein methylation has been most studied in histones, where the transfer of methyl groups from S-adenosyl methionine is catalyzed by histone methyltransferases. Histones that are methylated on certain residues can act epigenetically to repress or activate gene expression.

Arginylation is a post-translational modification in which proteins are modified by the addition of arginine (Arg) at the N-terminal amino group or side chains of reactive amino acids by the enzyme, arginyltransferase (ATE1). Recent studies have also revealed that hundreds of proteins in vivo are arginylated, proteins which are essential for many biological pathways. While still poorly understood in a biological setting, the ATE1 enzyme is highly conserved which suggests that arginylation is an important biological post-translational modification.













