Related Research Articles
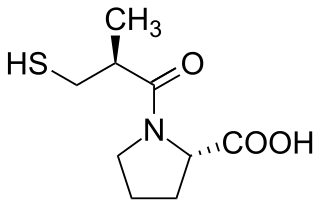
Angiotensin-converting-enzyme inhibitors are a class of medication used primarily for the treatment of high blood pressure and heart failure. They work by causing relaxation of blood vessels as well as a decrease in blood volume, which leads to lower blood pressure and decreased oxygen demand from the heart.

The renin–angiotensin system (RAS), or renin–angiotensin–aldosterone system (RAAS), is a hormone system that regulates blood pressure and fluid and electrolyte balance, as well as systemic vascular resistance.

Angiotensin is a peptide hormone that causes vasoconstriction and an increase in blood pressure. It is part of the renin–angiotensin system, which regulates blood pressure. Angiotensin also stimulates the release of aldosterone from the adrenal cortex to promote sodium retention by the kidneys.

Angiotensin-converting enzyme, or ACE, is a central component of the renin–angiotensin system (RAS), which controls blood pressure by regulating the volume of fluids in the body. It converts the hormone angiotensin I to the active vasoconstrictor angiotensin II. Therefore, ACE indirectly increases blood pressure by causing blood vessels to constrict. ACE inhibitors are widely used as pharmaceutical drugs for treatment of cardiovascular diseases.
The angiotensin II receptors, (ATR1) and (ATR2), are a class of G protein-coupled receptors with angiotensin II as their ligands. They are important in the renin–angiotensin system: they are responsible for the signal transduction of the vasoconstricting stimulus of the main effector hormone, angiotensin II.

Membrane alanyl aminopeptidase also known as alanyl aminopeptidase (AAP) or aminopeptidase N (AP-N) is an enzyme that in humans is encoded by the ANPEP gene.

Papain, also known as papaya proteinase I, is a cysteine protease enzyme present in papaya and mountain papaya. It is the namesake member of the papain-like protease family.

Angiotensin-converting enzyme 2 (ACE2) is an enzyme that can be found either attached to the membrane of cells (mACE2) in the intestines, kidney, testis, gallbladder, and heart or in a soluble form (sACE2). Both membrane bound and soluble ACE2 are integral parts of the renin–angiotensin–aldosterone system (RAAS) that exists to keep the body's blood pressure in check. While mACE2 does not appear to factor into the harmful phase of RAAS, its existence is vital in order for the enzyme ADAM17 to cleave its extracellular domain to create soluble ACE2 (sACE2). Soluble ACE2 lowers blood pressure by catalyzing the hydrolysis of angiotensin II into angiotensin (1–7) which in turns binds to MasR receptors creating localized vasodilation and hence decreasing blood pressure. This decrease in blood pressure makes the entire process a promising drug target for treating cardiovascular diseases.
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the amino terminus (N-terminus) of proteins or peptides (exopeptidases).They are widely distributed throughout the animal and plant kingdoms and are found in many subcellular organelles, in cytosol, and as membrane components. Aminopeptidases are used in essential cellular functions. Many, but not all, of these peptidases are zinc metalloenzymes.

Leucyl/cystinyl aminopeptidase, also known as cystinyl aminopeptidase (CAP), insulin-regulated aminopeptidase (IRAP), human placental leucine aminopeptidase (PLAP), oxytocinase, and vasopressinase, is an enzyme of the aminopeptidase group that in humans is encoded by the LNPEP gene.
Procollagen peptidase is an endopeptidase involved in the processing of collagen. The proteases removes the terminal peptides of the procollagen. Deficiency of these enzymes leads to dermatosparaxis or Ehlers–Danlos syndrome.

N-Acetylaspartylglutamic acid is a peptide neurotransmitter and the third-most-prevalent neurotransmitter in the mammalian nervous system. NAAG consists of N-acetylaspartic acid (NAA) and glutamic acid coupled via a peptide bond.

Dipeptidyl-peptidase 3 is an enzyme that in humans is encoded by the DPP3 gene.

Lysosomal Pro-X carboxypeptidase is an enzyme that in humans is encoded by the PRCP gene.

Chromosome 9 open reading frame 3 (C9ORF3) also known as aminopeptidase O (APO) is an enzyme which in humans is encoded by the C9ORF3 gene. The protein encoded by this gene is an aminopeptidase which is most closely related in sequence to leukotriene A4 hydrolase (LTA4H). APO is a member of the M1 metalloproteinase family.
Enkephalinases are enzymes that degrade endogenous enkephalin opioid peptides. They include:

Kelatorphan is a drug which acts as a powerful and complete inhibitor of nearly all of the enzymes responsible for catabolism of the endogenous enkephalins, including neutral endopeptidase (NEP), dipeptidyl peptidase III (DPP3), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE). In mice, with the intracerebroventricular co-administration of a 50 µg dose of kelatorphan (this route is necessary because kelatorphan is incapable of crossing the blood-brain-barrier) hence alongside exogenous [Met]enkephalin (ED50 approximately 10 ng), it potentiated the analgesic effects of the latter by 50,000 times. Kelatorphan also displays potent antinociceptive effects alone, and does not depress respiration, although at high doses it actually increases it.
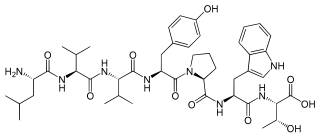
Spinorphin is an endogenous, non-classical opioid peptide of the hemorphin family first isolated from the bovine spinal cord (hence the prefix spin-) and acts as a regulator of the enkephalinases, a class of enzymes that break down endogenous the enkephalin peptides. It does so by inhibiting the enzymes aminopeptidase N (APN), dipeptidyl peptidase III (DPP3), angiotensin-converting enzyme (ACE), and neutral endopeptidase (NEP). Spinorphin is a heptapeptide and has the amino acid sequence Leu-Val-Val-Tyr-Pro-Trp-Thr (LVVYPWT). It has been observed to possess antinociceptive, antiallodynic, and anti-inflammatory properties. The mechanism of action of spinorphin has not been fully elucidated (i.e., how it acts to inhibit the enkephalinases), but it has been found to act as an antagonist of the P2X3 receptor, and as a weak partial agonist/antagonist of the FP1 receptor.

Tynorphin is a synthetic opioid peptide which is a potent and competitive inhibitor of the enkephalinase class of enzymes which break down the endogenous enkephalin peptides. It specifically inactivates dipeptidyl aminopeptidase III (DPP3) with very high efficacy, but also inhibits neutral endopeptidase (NEP), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE) to a lesser extent. It has a pentapeptide structure with the amino acid sequence Val-Val-Tyr-Pro-Trp (VVYPW).
Pyroglutamyl-peptidase II is an enzyme. This enzyme catalyses the following chemical reaction
References
- ↑ Reaux A, Iturrioz X, Vazeux G, Fournie-Zaluski MC, David C, Roques BP, Corvol P, Llorens-Cortes C (2000). "Aminopeptidase A, which generates one of the main effector peptides of the brain renin-angiotensin system, angiotensin III, has a key role in central control of arterial blood pressure". Biochem. Soc. Trans. 28 (4): 435–40. doi:10.1042/0300-5127:0280435. PMID 10961935.