Dystroglycan is a protein that in humans is encoded by the DAG1 gene.

Galectins are a class of proteins that bind specifically to β-galactoside sugars, such as N-acetyllactosamine, which can be bound to proteins by either N-linked or O-linked glycosylation. They are also termed S-type lectins due to their dependency on disulphide bonds for stability and carbohydrate binding. There have been about 15 galectins discovered in mammals, encoded by the LGALS genes, which are numbered in a consecutive manner. Only galectin-1, -2, -3, -4, -7, -7B, -8, -9, -9B, 9C, -10, -12, -13, -14, and -16 have been identified in humans. Galectin-5 and -6 are found in rodents, whereas galectin-11 and -15 are uniquely found in sheep and goats. Members of the galectin family have also been discovered in other mammals, birds, amphibians, fish, nematodes, sponges, and some fungi. Unlike the majority of lectins they are not membrane bound, but soluble proteins with both intra- and extracellular functions. They have distinct but overlapping distributions but found primarily in the cytosol, nucleus, extracellular matrix or in circulation. Although many galectins must be secreted, they do not have a typical signal peptide required for classical secretion. The mechanism and reason for this non-classical secretion pathway is unknown.

CD68 is a protein highly expressed by cells in the monocyte lineage, by circulating macrophages, and by tissue macrophages.
The epididymal secretory protein E1, also known as NPC2( Niemann-Pick intracellular cholesterol transporter 2), is one of two main lysosomal transport proteins that assist in the regulation of cellular cholesterol by exportation of LDL-derived cholesterol from lysosomes. Lysosomes have digestive enzymes that allow it to break down LDL particles to LDL-derived cholesterol once the LDL particle is engulfed into the cell via receptor mediated endocytosis.
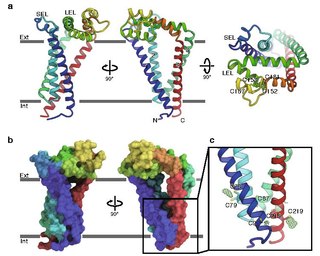
CD9 is a gene encoding a protein that is a member of the transmembrane 4 superfamily also known as the tetraspanin family. It is a cell surface glycoprotein that consists of four transmembrane regions and has two extracellular loops that contain disulfide bonds which are conserved throughout the tetraspanin family. Also containing distinct palmitoylation sites that allows CD9 to interact with lipids and other proteins.

CD63 antigen is a protein that, in humans, is encoded by the CD63 gene. CD63 is mainly associated with membranes of intracellular vesicles, although cell surface expression may be induced.

Mitochondrial uncoupling protein 3 is a protein that in humans is encoded by the UCP3 gene. The gene is located in chromosome (11q13.4) with an exon count of 7 and is expressed on the inner mitochondrial membrane. Uncoupling proteins transfer anions from the inner mitochondrial membrane to the outer mitochondrial membrane, thereby separating oxidative phosphorylation from synthesis of ATP, and dissipating energy stored in the mitochondrial membrane potential as heat. Uncoupling proteins also reduce generation of reactive oxygen species.
Ras-related protein Rab-7a is a protein that in humans is encoded by the RAB7A gene.
HLA class II histocompatibility antigen, DM beta chain is a protein that in humans is encoded by the HLA-DMB gene.

Tripeptidyl-peptidase 1, also known as Lysosomal pepstatin-insensitive protease, is an enzyme that in humans is encoded by the TPP1 gene. TPP1 should not be confused with the TPP1 shelterin protein which protects telomeres and is encoded by the ACD gene. Mutations in the TPP1 gene leads to late infantile neuronal ceroid lipofuscinosis.

Lysosomal-associated membrane protein 1 (LAMP-1) also known as lysosome-associated membrane glycoprotein 1 and CD107a, is a protein that in humans is encoded by the LAMP1 gene. The human LAMP1 gene is located on the long arm (q) of chromosome 13 at region 3, band 4 (13q34).

CTNS may also refer to the Center for Theology and the Natural Sciences.

HLA class II histocompatibility antigen, DO alpha chain is a protein that in humans is encoded by the HLA-DOA gene.

HLA class II histocompatibility antigen, DO beta chain is a protein that in humans is encoded by the HLA-DOB gene.
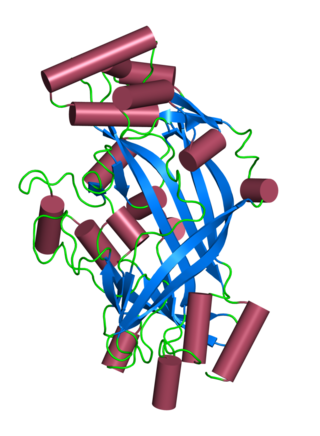
Lysosomal integral membrane protein 2 (LIMP-2) is a protein that in humans is encoded by the SCARB2 gene. LIMP-2 is expressed in brain, heart, liver, lung and kidney, mainly in the membrane of lysosome organelles; however, in cardiac muscle, LIMP-2 is also expressed at intercalated discs. LIMP-2 in a membrane protein in lysosomes that functions to regulate lysosomal/endosomal transport. Mutations in LIMP-2 have been shown to cause Gaucher disease, myoclonic epilepsy, and action myoclonus–renal failure syndrome. Abnormal levels of LIMP-2 have also been found in patients with hypertrophic cardiomyopathy.

Collagen alpha-1(XV) chain is a protein that in humans is encoded by the COL15A1 gene.

Lysosome-associated membrane glycoprotein 3 is a protein that in humans is encoded by the LAMP3 gene. It is one of the lysosome-associated membrane glycoproteins.
Lysosome-associated membrane glycoproteins (LAMPs) are integral membrane proteins, specific to lysosomes, and whose exact biological function is not yet clear. Structurally, the lamp proteins consist of two internally homologous lysosome-luminal domains separated by a proline-rich hinge region; at the C-terminal extremity there is a transmembrane region (TM) followed by a very short cytoplasmic tail (C). In each of the duplicated domains, there are two conserved disulfide bonds. This structure is schematically represented in the figure below.
+-----+ +-----+ +-----+ +-----+ | | | | | | | | xCxxxxxCxxxxxxxxxxxxCxxxxxCxxxxxxxxxCxxxxxCxxxxxxxxxxxxCxxxxxCxxxxxxxx +--------------------------++Hinge++--------------------------++TM++C+

Chaperone-mediated autophagy (CMA) refers to the chaperone-dependent selection of soluble cytosolic proteins that are then targeted to lysosomes and directly translocated across the lysosome membrane for degradation. The unique features of this type of autophagy are the selectivity on the proteins that are degraded by this pathway and the direct shuttling of these proteins across the lysosomal membrane without the requirement for the formation of additional vesicles.

ADP/ATP translocase 2 is a protein that in humans is encoded by the SLC25A5 gene on the X chromosome.