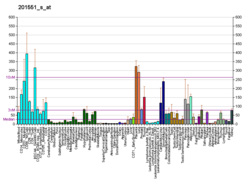
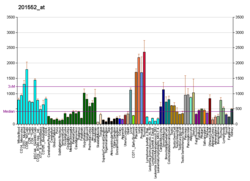
Cathepsin S is a protein that in humans is encoded by the CTSS gene. Transcript variants utilizing alternative polyadenylation signals exist for this gene.

Acid alpha-glucosidase, also called acid maltase, is an enzyme that helps to break down glycogen in the lysosome. It is functionally similar to glycogen debranching enzyme, but is on a different chromosome, processed differently by the cell and is located in the lysosome rather than the cytosol. In humans, it is encoded by the GAA gene. Errors in this gene cause glycogen storage disease type II.

Beta-hexosaminidase subunit beta is an enzyme that in humans is encoded by the HEXB gene.

Osteonectin (ON) also known as secreted protein acidic and rich in cysteine (SPARC) or basement-membrane protein 40 (BM-40) is a protein that in humans is encoded by the SPARC gene.

CD68 is a protein highly expressed by cells in the monocyte lineage, by circulating macrophages, and by tissue macrophages.
The mannose 6-phosphate receptors (MPRs) are transmembrane glycoproteins that target enzymes to lysosomes in vertebrates.
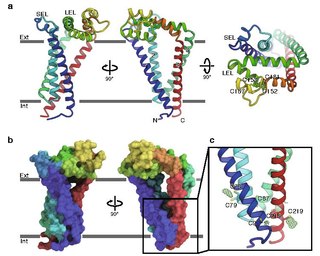
CD9 is a gene encoding a protein that is a member of the transmembrane 4 superfamily also known as the tetraspanin family. It is a cell surface glycoprotein that consists of four transmembrane regions and has two extracellular loops that contain disulfide bonds which are conserved throughout the tetraspanin family. Also containing distinct palmitoylation sites that allows CD9 to interact with lipids and other proteins.

CD63 antigen is a protein that, in humans, is encoded by the CD63 gene. CD63 is mainly associated with membranes of intracellular vesicles, although cell surface expression may be induced.

Lysosome-associated membrane protein 2 (LAMP2), also known as CD107b and Mac-3, is a human gene. Its protein, LAMP2, is one of the lysosome-associated membrane glycoproteins.

Cathepsin D is a protein that in humans is encoded by the CTSD gene. This gene encodes a lysosomal aspartyl protease composed of a protein dimer of disulfide-linked heavy and light chains, both produced from a single protein precursor. Cathepsin D is an aspartic endo-protease that is ubiquitously distributed in lysosomes. The main function of cathepsin D is to degrade proteins and activate precursors of bioactive proteins in pre-lysosomal compartments. This proteinase, which is a member of the peptidase A1 family, has a specificity similar to but narrower than that of pepsin A. Transcription of the CTSD gene is initiated from several sites, including one that is a start site for an estrogen-regulated transcript. Mutations in this gene are involved in the pathogenesis of several diseases, including breast cancer and possibly Alzheimer disease. Homozygous deletion of the CTSD gene leads to early lethality in the postnatal phase. Deficiency of CTSD gene has been reported an underlying cause of neuronal ceroid lipofuscinosis (NCL).

Granulin is a protein that in humans is encoded by the GRN gene. Each granulin protein is cleaved from the precursor progranulin, a 593 amino-acid-long and 68.5 kDa protein. While the function of progranulin and granulin have yet to be determined, both forms of the protein have been implicated in development, inflammation, cell proliferation and protein homeostasis. The 2006 discovery of the GRN mutation in a population of patients with frontotemporal dementia has spurred much research in uncovering the function and involvement in disease of progranulin in the body. While there is a growing body of research on progranulin's role in the body, studies on specific granulin residues are still limited.

Plasma membrane calcium-transporting ATPase 4 is an enzyme that in humans is encoded by the ATP2B4 gene.
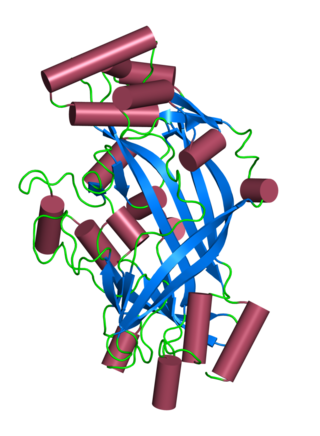
Lysosomal integral membrane protein 2 (LIMP-2) is a protein that in humans is encoded by the SCARB2 gene. LIMP-2 is expressed in brain, heart, liver, lung and kidney, mainly in the membrane of lysosome organelles; however, in cardiac muscle, LIMP-2 is also expressed at intercalated discs. LIMP-2 in a membrane protein in lysosomes that functions to regulate lysosomal/endosomal transport. Mutations in LIMP-2 have been shown to cause Gaucher disease, myoclonic epilepsy, and action myoclonus–renal failure syndrome. Abnormal levels of LIMP-2 have also been found in patients with hypertrophic cardiomyopathy.

Synaptotagmin-2 is a protein that in humans is encoded by the SYT2 gene.

Alpha-2,8-sialyltransferase 8B is an enzyme that in humans is encoded by the ST8SIA2 gene.

Lysosome-associated membrane glycoprotein 3 is a protein that in humans is encoded by the LAMP3 gene. It is one of the lysosome-associated membrane glycoproteins.

Alpha-(1,3)-fucosyltransferase is an enzyme that in humans is encoded by the FUT5 gene.
Lysosome-associated membrane glycoproteins (LAMPs) are integral membrane proteins, specific to lysosomes, and whose exact biological function is not yet clear. Structurally, the lamp proteins consist of two internally homologous lysosome-luminal domains separated by a proline-rich hinge region; at the C-terminal extremity there is a transmembrane region (TM) followed by a very short cytoplasmic tail (C). In each of the duplicated domains, there are two conserved disulfide bonds. This structure is schematically represented in the figure below.
+-----+ +-----+ +-----+ +-----+ | | | | | | | | xCxxxxxCxxxxxxxxxxxxCxxxxxCxxxxxxxxxCxxxxxCxxxxxxxxxxxxCxxxxxCxxxxxxxx +--------------------------++Hinge++--------------------------++TM++C+

Chaperone-mediated autophagy (CMA) refers to the chaperone-dependent selection of soluble cytosolic proteins that are then targeted to lysosomes and directly translocated across the lysosome membrane for degradation. The unique features of this type of autophagy are the selectivity on the proteins that are degraded by this pathway and the direct shuttling of these proteins across the lysosomal membrane without the requirement for the formation of additional vesicles.
The lysosomal cystine transporter (LCT) family is part of the TOG Superfamily and includes secondary transport proteins that are derived from animals, plants, fungi and other eukaryotes. They exhibit 7 putative transmembrane α-helical spanners (TMSs) and vary in size between about 200 and 500 amino acyl residues, although most have between 300 and 400 residues.