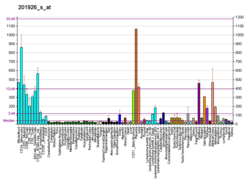
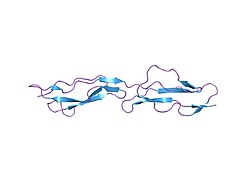
Complement decay-accelerating factor, also known as CD55 or DAF, is a protein that, in humans, is encoded by the CD55 gene. [5]
DAF regulates the complement system on the cell surface. It recognizes C4b and C3b fragments that are created during activation of C4 (classical or lectin pathway) or C3 (alternative pathway). Interaction of DAF with cell-associated C4b of the classical and lectin pathways interferes with the conversion of C2 to C2b, thereby preventing formation of the C4b2a C3-convertase, and interaction of DAF with C3b of the alternative pathway interferes with the conversion of factor B to Bb by factor D, thereby preventing formation of the C3bBb C3 convertase of the alternative pathway. Thus, by limiting the amplification convertases of the complement cascade, DAF indirectly blocks the formation of the membrane attack complex. [6]
This glycoprotein is broadly distributed among hematopoietic and non-hematopoietic cells. It is a determinant for the Cromer blood group system.