The Epstein–Barr virus (EBV), formally called Human gammaherpesvirus 4, is one of the nine known human herpesvirus types in the herpes family, and is one of the most common viruses in humans. EBV is a double-stranded DNA virus. Epstein–Barr virus (EBV) is the first identified oncogenic virus, that is a virus that can cause cancer. EBV establishes permanent infection in humans. It causes infectious mononucleosis and is also tightly linked to many malignant diseases (cancers). Various vaccine formulations underwent testing in different animals or in humans. However, none of them were able to prevent EBV infection and no vaccine has been approved to date.

Complement receptor type 1 (CR1) also known as C3b/C4b receptor or CD35 is a protein that in humans is encoded by the CR1 gene.

CD23, also known as Fc epsilon RII, or FcεRII, is the "low-affinity" receptor for IgE, an antibody isotype involved in allergy and resistance to parasites, and is important in regulation of IgE levels. Unlike many of the antibody receptors, CD23 is a C-type lectin. It is found on mature B cells, activated macrophages, eosinophils, follicular dendritic cells, and platelets.
Co-stimulation is a secondary signal which immune cells rely on to activate an immune response in the presence of an antigen-presenting cell. In the case of T cells, two stimuli are required to fully activate their immune response. During the activation of lymphocytes, co-stimulation is often crucial to the development of an effective immune response. Co-stimulation is required in addition to the antigen-specific signal from their antigen receptors.

CD11c, also known as Integrin, alpha X (ITGAX), is a gene that encodes for CD11c.
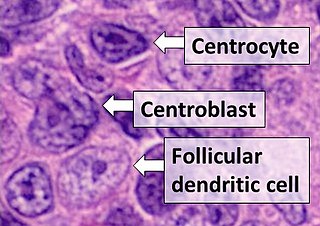
Follicular dendritic cells (FDC) are cells of the immune system found in primary and secondary lymph follicles of the B cell areas of the lymphoid tissue. Unlike dendritic cells (DC), FDCs are not derived from the bone-marrow hematopoietic stem cell, but are of mesenchymal origin. Possible functions of FDC include: organizing lymphoid tissue's cells and microarchitecture, capturing antigen to support B cell, promoting debris removal from germinal centers, and protecting against autoimmunity. Disease processes that FDC may contribute include primary FDC-tumor, chronic inflammatory conditions, HIV-1 infection development, and neuroinvasive scrapie.

B-lymphocyte antigen CD19, also known as CD19 molecule, B-Lymphocyte Surface Antigen B4, T-Cell Surface Antigen Leu-12 and CVID3 is a transmembrane protein that in humans is encoded by the gene CD19. In humans, CD19 is expressed in all B lineage cells. Contrary to some early doubts, human plasma cells do express CD19. CD19 plays two major roles in human B cells: on the one hand, it acts as an adaptor protein to recruit cytoplasmic signaling proteins to the membrane; on the other, it works within the CD19/CD21 complex to decrease the threshold for B cell receptor signaling pathways. Due to its presence on all B cells, it is a biomarker for B lymphocyte development, lymphoma diagnosis and can be utilized as a target for leukemia immunotherapies.
Macrophage-1 antigen is a complement receptor ("CR3") consisting of CD11b and CD18.
Angioimmunoblastic T-cell lymphoma is a mature T-cell lymphoma of blood or lymph vessel immunoblasts characterized by a polymorphous lymph node infiltrate showing a marked increase in follicular dendritic cells (FDCs) and high endothelial venules (HEVs) and systemic involvement.

C-C chemokine receptor type 7 is a protein that in humans is encoded by the CCR7 gene. Two ligands have been identified for this receptor: the chemokines ligand 19 (CCL19/ELC) and ligand 21 (CCL21). The ligands have similar affinity for the receptor, though CCL19 has been shown to induce internalisation of CCR7 and desensitisation of the cell to CCL19/CCL21 signals. CCR7 is a transmembrane protein with 7 transmembrane domains, which is coupled with heterotrimeric G proteins, which transduce the signal downstream through various signalling cascades. The main function of the receptor is to guide immune cells to immune organs by detecting specific chemokines, which these tissues secrete.

T-cell surface glycoprotein CD3 gamma chain is a protein that in humans is encoded by the CD3G gene.

Annexin A6 is a protein that in humans is encoded by the ANXA6 gene.

CD81 molecule, also known as CD81, is a protein which in humans is encoded by the CD81 gene. It is also known as 26 kDa cell surface protein, TAPA-1, and Tetraspanin-28 (Tspan-28).

SLAM family member 6 is a protein that in humans is encoded by the SLAMF6 gene.

Epstein-Barr virus induced gene 3, also known as interleukin-27 subunit beta or IL-27B, is a protein which in humans is encoded by the EBI3 gene.

CD79b molecule, immunoglobulin-associated beta, also known as CD79B, is a human gene.
VG-1 is a B cell line which was derived from primary effusion lymphoma (PEL). It was first established in 2000 by David T. Scadden’s group at Massachusetts General Hospital. It is infected with Kaposi's sarcoma-associated herpesvirus (KSHV), but negative with Epstein–Barr virus (EBV).
Epstein–Barr virus (EBV) latent membrane protein 2 (LMP2) are two viral proteins of the Epstein–Barr virus. LMP2A/LMP2B are transmembrane proteins that act to block tyrosine kinase signaling. LMP2A is a transmembrane protein that inhibits normal B-cell signal transduction by mimicking an activated B-cell receptor (BCR). The N-terminus domain of LMP2A is tyrosine phosphorylated and associates with Src family protein tyrosine kinases (PTKs) as well as spleen tyrosine kinase (Syk). PTKs and Syk are associated with BCR signal transduction.
The NSG mouse is a brand of immunodeficient laboratory mice, developed and marketed by Jackson Laboratory, which carries the strain NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ. NSG branded mice are among the most immunodeficient described to date. NSG branded mice lack mature T cells, B cells, and natural killer (NK) cells. NSG branded mice are also deficient in multiple cytokine signaling pathways, and they have many defects in innate immunity. The compound immunodeficiencies in NSG branded mice permit the engraftment of a wide range of primary human cells, and enable sophisticated modeling of many areas of human biology and disease. NSG branded mice were developed in the laboratory of Dr. Leonard Shultz at Jackson Laboratory, which owns the NSG trade mark.
Sushi domain is an evolutionarily conserved protein domain. It is also known as complement control protein (CCP) modules or short consensus repeats (SCR). The name derives from the visual similarity of the domain to nigiri sushi when the primary structure is drawn showing the loops created by the disulfide bonds.