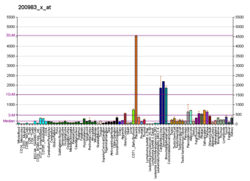
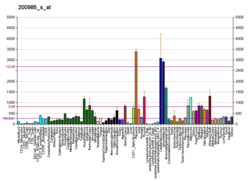
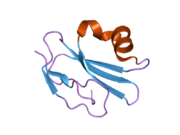
CD59 glycoprotein, also known as MAC-inhibitory protein (MAC-IP), membrane inhibitor of reactive lysis (MIRL), or protectin, is a protein that in humans is encoded by the CD59 gene. [5] It is an LU domain and belongs to the LY6/uPAR/alpha-neurotoxin protein family. [6]
CD59 attaches to host cells via a glycophosphatidylinositol (GPI) anchor. Cholesterol-containing microdomains aid in CD59 activity by stimulating a "pinch point" in the lipid membrane during MAC assembly to prevent pore-formation and inhibit lysing. [7] When complement activation leads to deposition of C5b678 on host cells, CD59 can prevent C9 from polymerizing and forming the complement membrane attack complex. [8] It may also signal the cell to perform active measures such as endocytosis of the CD59-C9 complex. [6] Endocytosis of this complex leads to the destruction of the ion channel formation that this complex provides to the MAC. These ion channels are used for transfer of different ions to maintain the correct concentration of minerals inside and outside of the membrane, and without this correct maintenance, severe symptoms and diseases can occur such as neuron degeneration and Alzheimer's disease. [9]
Mutations affecting GPI that reduce expression of CD59 and decay-accelerating factor on red blood cells result in paroxysmal nocturnal hemoglobinuria. [10] GPI mutation and consequent reduction in CD59 expression results from a cysteine to tyrosine missense mutation, which prevents disulfide bridge formation, ultimately disrupting tertiary protein structure and preventing proper GPI-CD59 complex binding. [11]
Viruses such as HIV, human cytomegalovirus and vaccinia incorporate host cell CD59 into their own viral envelope to prevent lysis by complement. [12] Additionally, CD59 has been investigated as a target for immunotherapy when treating certain cancers such as breast cancer. Researchers have found that once CD59 had been targeted, there is an upregulation in fas and caspase-3, creating an increase in apoptosis and tumor growth suppression in MCF-7 cells. [13]